Graphical abstract

Keywords: Spotted hyaena, Dipylidium, Molecular screening, Parasite infection, Grooming, Serengeti
Highlights
-
•
We investigated Dipylidium infection in a social carnivore, the spotted hyaena.
-
•
Infection predominantly occurred in juveniles, most adults were probably immune.
-
•
Juvenile infection prevalence increased with the number of hyaenas visiting dens.
-
•
Infection prevalence in juveniles decreased when they were least well fed.
-
•
The use of communal dens maintains Dipylidium infection in spotted hyaena clans.
Abstract
We provide the first genetic sequence data for a Dipylidium species from a wild carnivore plus an analysis of the effects of ecological, demographic, physiological and behavioural factors on Dipylidium sp. infection prevalence in a social carnivore, the spotted hyaena (Crocuta crocuta), in the Serengeti National Park, Tanzania. Our sequence data from a mitochondrial gene fragment (1176 base pair long) had a similarity of between 99% and 89% to Dipylidium caninum. We determined infection prevalence in 146 faecal samples from 124 known animals in three social groups (termed clans) using molecular screening and Dipylidium proglottid presence. Our analysis revealed significantly higher infection prevalence in juveniles (55%) than adults (15.8%), indicating that predominantly juveniles maintained infection in clans. The likelihood of infection in juveniles significantly: (1) increased as the number of adults and older juveniles (>6 months) at communal dens increased, implying a positive relationship between this factor and the size of the intermediate host (probably a flea species) population at communal dens; (2) decreased as the number of younger juveniles (<6 months) increased, suggesting that the chance of susceptible juveniles ingesting infected fleas during self-grooming declined as the number of infected fleas per younger juvenile declined; and (3) decreased during periods of low prey abundance in clan territories when an increased reliance on long-distances foraging excursions reduces the number of clan members visiting communal dens, possibly resulting in a decline in flea populations at dens. Long-distance foraging also increases the intervals (in days) between nursing visits by lactating females to their offspring. Lengthy intervals between milk intake by infected juveniles may reduce adult Dipylidium fecundity and hence decrease infection prevalence in the den flea population. Our study provides useful insights into Dipylidium epidemiology in a social carnivore population subject to large fluctuations in prey abundance.
1. Introduction
The adult form of Dipylidium caninum is an intestinal parasite with a worldwide distribution in the domestic dog (Canis familiaris) and domestic cat (Felis catus). Occasional cases of infection in humans, particularly children, can occur (e.g., Wong, 1955; Molina et al., 2003). This parasite (or a closely related Dipylidium species) also infects wild carnivores in the families Canidae and Hyaenidae (Table 1) and infection prevalence varies both within and between host species (Table 1).
Table 1.
Infection prevalence of Dipylidium caninum (or Dipylidium sp.) in domestic and wild carnivores (N indicates number of samples screened).
| Species | Scientific name | Family | Prevalence (%) | N | Method | Location | Reference |
|---|---|---|---|---|---|---|---|
| Domestic dog | Canis familiaris | Canidae | 0.1 | 1400 | Coprology | Australia | Palmer et al. (2008) |
| Domestic dog | Canis familiaris | Canidae | 0.1 | 8438 | Coprology | Germany | Barutzki and Schaper (2003) |
| Domestic dog | Canis familiaris | Canidae | 0.7 | 3780 | Coprology | Czech Republic | Dubná et al. (2007) |
| Domestic dog | Canis familiaris | Canidae | 0.7 | 271 | Coprology | Brazil | Oliveira-Sequeira et al. (2002) |
| Domestic dog | Canis familiaris | Canidae | 0.8 | 2193 | Coprology | Argentina | Fontanarrossa et al. (2006) |
| Domestic dog | Canis familiaris | Canidae | 1.3 | 450 | Coprology | Czech Republic | Dubná et al. (2007) |
| Domestic dog | Canis familiaris | Canidae | 2.2 | 540 | Coprology | Zambia | Nonaka et al. (2011) |
| Domestic dog | Canis familiaris | Canidae | 8.9 | 45 | Coprology | Brazil | Santos et al. (2012) |
| Domestic dog | Canis familiaris | Canidae | 11.9 | 841 | Coprology & necropsy | Spain | Martinez-Moreno et al. (2007) |
| Domestic dog | Canis familiaris | Canidae | 14.7 | 366 | Coprology & necropsy | Spain | Martinez-Moreno et al. (2007) |
| Domestic dog | Canis familiaris | Canidae | 44.0 | 63 | Coprology & necropsy | South Africa | Minnaar et al. (2002) |
| Domestic dog | Canis familiaris | Canidae | 38.5 | 83 | Necropsy | Iran | Dalimi et al. (2006) |
| Domestic dog | Canis familiaris | Canidae | 60.0 | 102 | Necropsy | Mexico | Eguia-Aguilar et al. (2005) |
| Domestic cat | Felis catus | Felidae | 0.03 | 3167 | Coprology | Germany | Barutzki and Schaper (2003) |
| Domestic cat | Felis catus | Felidae | 0.2 | 1063 | Coprology | Australia | Palmer et al. (2008) |
| Domestic cat | Felis catus | Felidae | 5.0 | 113 | Coprology | Egypt | Khalafalla (2011) |
| Domestic cat | Felis catus | Felidae | 20.7 | 58 | Necropsy | Spain | Calvete et al. (1998) |
| Domestic cat | Felis catus | Felidae | 34.8 | 92 | Necropsy | England | Nichol et al. (1981) |
| Domestic cat | Felis catus | Felidae | 52.6 | 135 | Necropsy | Brazil | Labarthe et al. (2004) |
| Grey wolf | Canis lupus | Canidae | 6.4 | 47 | Necropsy | Spain | Segovia et al. (2001) |
| Red fox | Vulpes vulpes | Canidae | 2.0 | 280 | Necropsy | Wales | Hackett and Walters (1980) |
| Red fox | Vulpes vulpes | Canidae | 3.8 | 843 | Necropsy | England | Richards et al. (1995) |
| Red fox | Vulpes vulpes | Canidae | 9.1 | 22 | Necropsy | Iran | Dalimi et al. (2006) |
| Red fox | Vulpes vulpes | Canidae | 57.3 | 129 | Necropsy | Italy | Magi et al., 2009 |
| Golden jackal | Canis aureus | Canidae | 0.0 | 10 | Necropsy | Iran | Dalimi et al. (2006) |
| Golden jackal | Canis aureus | Canidae | Present | 5 | Necropsy | Kenya | Nelson et al. (1965) |
| Side-striped jackal | Canis adustus | Canidae | Present | 2 | Necropsy | Kenya | Nelson et al. (1965) |
| Silver-backed jackal | Canis mesomelas | Canidae | Present | 9 | Necropsy | Kenya | Nelson et al. (1965) |
| Crab-eating fox | Cerdocyon thous | Canidae | 20.0 | 5 | Coprology | Brazil | Santos et al. (2012) |
| Maned wolf | Chrysocyon brachyurus | Canidae | 0.0 | 33 | Coprology | Brazil | Nelson et al. (1965) |
| Spotted hyaena | Crocuta crocuta | Hyaenidae | 21.4 | 70 | Coprology | Kenya | Engh et al. (2003) |
| African lion | Panthera leo | Felidae | 0.0 | 112 | Coprology | Tanzania | Müller-Graf (1995); Bjork et al. (2000) |
Adult D. caninum shed proglottids (containing packets of eggs) which can be visible on the surface of faeces. Fleas in the genus Ctenocephalides are considered the main intermediate hosts of D. caninum but fleas in the genus Pulex and the dog louse Trichodectes canis can also act as intermediate hosts (Pugh, 1987). Eggs of D. caninum are ingested by flea larvae and D. caninum larvae then hatch and migrate into the body cavity. Infective cysticercoid larvae develop when adult fleas emerge from their cocoons and encounter the high body temperature of a mammalian host (Pugh, 1987). Mammalian hosts are infected when they ingest infected fleas, for example during grooming. Mammalian hosts infected with D. caninum may have a high worm burden (e.g., up to 130 adult worms) because larval fleas typically ingest whole worm packets, resulting in the development of multiple cysticercoids per flea (Nichol et al., 1981).
In domestic carnivores, D. caninum infection prevalence is higher among younger than older animals (e.g., Fontanarrossa et al., 2006; Martinez-Moreno et al., 2007) and rural, stray and feral animals are more often infected than individually owned and responsibly cared for animals in urban environments (e.g. Dubná et al., 2007; Martinez-Moreno et al., 2007). Factors influencing infection by Dipylidium spp. in wild carnivores (see studies quoted in Table 1) are less well known.
Whether ingestion of an infected flea results in the development of an adult Dipylidium spp. parasite may depend on the quality of the immune response of the mammalian host to infection. Studies on mammalian host immune responses to Taenia species (reviewed by Lightowlers et al., 2003) and of rodents to Hymenolepis diminuta infection (reviewed by McKay, 2010) have revealed complex host immune responses to cestode infection and evidence for the development of immunological memory following an initial infection with some but not all cestodes. Furthermore, cross immunity between cestode species may occur, as previous and concurrent infection with one species can prevent infection with another species (e.g., Gabriele et al., 1988; Ito et al., 1988; Wang and McKay, 2005). There is evidence that acquisition of immunity against helminth parasites may be impaired by severe undernourishment, particularly in hosts that lose protein mass (reviewed by Coop and Kyriazakis, 1999), possibly because of tradeoffs between the allocation of body resources to immunity and other key body functions (Coop and Kyriazakis, 1999; Schmid-Hempel, 2011).
Challenging environmental conditions can increase the energetic cost of maintaining homeostasis. The cumulative energetic cost of this is termed allostatic load (McEwen and Wingfield, 2010). When allostatic load increases, glucocorticoid secretion is typically elevated (reviewed by Goymann and Wingfield, 2004) as recently found in juvenile spotted hyaena (Benhaiem et al., 2013). When glucocorticoid concentrations are chronically elevated they can also reduce immunocompetence (Wingfield et al., 1998; Hofer and East, 2012).
In East Africa, the spotted hyaena is infected with Dipylidium sp. (Engh et al., 2003, Table 1). Neither the genetic identity of this parasite nor the factors influencing infection in spotted hyaenas are known. Our study aimed to provide the first genetic information for the Dipylidium species infecting this host in East Africa and to investigate ecological, demographic, behavioural and physiological factors that may influence Dipylidium infection in this social carnivore. We assumed the intermediate host of this Dipylidium species is probably a flea (Insecta, Siphonaptera) and possibly the ‘stick fast flea’ Echidnophaga larina. Adult stick fast fleas live attached to the skin of their host. E. larina was found on nine spotted hyaenas examined in the Kruger National Park, South Africa (Horak et al., 2004) and a species of stick fast fleas occur on spotted hyaenas in our study population. There is also evidence from Kenya that E. larina from an unspecified jackal species carry Dipylidium cysticercoids in Kenya (Spinage, 2012).
The spotted hyaena is a social carnivore which forms multi-female, multi-male clans (Kruuk, 1972; Frank, 1986a; East and Hofer, 2013). Clans are fission fusion societies in which individual members or small groups operate independently and clan members only congregate at large carcasses (Kruuk, 1972; Frank, 1986a), or when called to cooperate in activities such as boundary disputes with neighbouring clans (East and Hofer, 1991). All adult females reproduce and rear their offspring at a communal den inside the clan’s territory (Kruuk, 1972). Individuals of both sexes visit communal dens (Fig. 1a) to socialise with other clan members (Smale et al., 1993) and scent-mark (East et al., 2013).
Fig. 1.

(a) A spotted hyaena communal den. (b) Dipylidium sp. egg capsule obtained from a gravid proglottid collected from a spotted hyaena faeces. (c) Spotted hyaena faeces with Dipylidium proglottids present on the surface.
Information on flea biology (reviewed by Dryden and Rust, 1994; Bitam et al., 2010) suggests that spotted hyaena communal dens are the location within a clan’s territory likely to contain a large part of the intermediate host population (in terms of adult fleas and their larvae) of Dipylidium because flea infestations are known to accumulate at sites frequently used by mammal hosts. Hence we assume that the non-adhesive eggs produced by fleas infesting spotted hyaenas at clan communal dens (including animals visiting dens and juveniles based at dens) fall onto soil in the den area (Fig 1a) and into underground burrows or are removed during grooming, and that the accumulation of eggs in the den vicinity increases with the number of clan members present at communal dens. Flea egg development requires a microenvironment in which temperature and relative humidity should remain within certain limits. We suspect this rarely occurs above ground but is likely in underground burrows of the communal den. Moreover, as flea larvae avoid light and move towards gravity, and are susceptible to desiccation, larvae that hatch or move into burrows are possibly more likely to survive than those exposed to desiccation and high temperatures above ground (see Dryden and Rust, 1994; Bitam et al., 2010). Hence communal den areas are likely to be locations with an accumulation of flea eggs and underground burrows of dens provide a microhabitat conducive to flea laval development.
Our study was conducted in the Serengeti National Park in northern Tanzania, where clans have the same social structure as populations elsewhere (East and Hofer, 2013) and defend territories of approximately 56 km2 (Hofer and East, 1995). The foraging behaviour of spotted hyaenas in the Serengeti National Park is unusual in that it has evolved to permit year-round consumption of migratory herbivores, mostly wildebeest (Connochaetes taurinus), Thomson’s gazelle (Gazella thomsoni), and plains zebra (Equus quagga) (Hofer and East, 1993a,b). When prey abundance is low in a clan’s territory, all migratory herbivores are absent and only resident prey is present. During these periods, solitary clan members or small groups travel long-distances on round trips of between 80 and 140 km (termed commuting trips) to locate large congregations of migratory prey on which to feed before returning to their home territory (Hofer and East, 1993a,b). When a high abundance of migratory prey is present in a clan’s territory, all clan members forage in their home territory (Hofer and East, 1993c). During periods between these two extremes, when only small herds of migratory prey are present in a clan’s territory, commuting frequency is determined by an individual’s social status. Commuting trips of lactating females last between 2 and 9 day (Hofer and East, 1993c).
Commuting behaviour is likely to influence Dipylidium infection in several ways. Firstly, commuting reduces the number of clan members visiting communal dens (Hofer and East, 1993c), which would be expected to lead to a reduction in the size of the communal den flea population. Secondly, commuting increases the period between nursing bouts for dependent juveniles at communal dens and can significantly reduce their milk intake (Hofer and East, 1993c, 2008). We expected the reduced nutritional status of juveniles during commuting periods to compromise juvenile immune responses and hence increase infection prevalence. Juveniles show considerable variation in faecal glucocorticoid metabolite concentrations (fGMCs) ranging between 4.6 and 377.4 ng/g (Benhaiem et al., 2012), and those experiencing challenging social and physical environments have elevated fGMCs, indicative of an elevated allostatic load (Benhaiem et al., 2013). We expected highly elevated fGMCs to increase prevalence of Dipylidium infection because of allocation trade-offs in the investment of body resources in immunity and maintenance.
Female spotted hyaenas with high social status have priority of access to food resources in their clan territory (Kruuk, 1972; Frank, 1986b). Offspring with mothers of high social status have higher growth rates (Hofer and East, 2003) and survival (Holekamp et al., 1996; Hofer and East, 2003) than offspring of female with low social status. Hence high-born offspring should less often need to trade-off investment in growth and immunity during their development, and have lower prevalence of Dipylidium infection than low-born offspring.
In several previous studies for which we collected spotted hyaena faeces (e.g., Goymann et al., 2001; East et al., 2004; Goller et al., 2013) we often observed Dipylidium proglottids on faeces from juveniles but more rarely on faeces from adults. For this reason, in this study we predicted a higher infection prevalence in juveniles than in adults and hence focused our study on factors likely to influence infection in juveniles. Our general expectations were that infection prevalence in spotted hyaenas would be modulated by factors influencing the population size of the intermediate flea host and the susceptibility of spotted hyaenas to infection by adult worms.
Spotted hyaenas can only be infected with Dipylidium when they consume an infected adult flea. We suspect that fleas are mostly consumed when animals groom either themselves or other clan members, such as mothers grooming their offspring. Grooming consists of vigorous licking and ‘nibbling’ of fur or skin with incisors. Licking removes flea eggs and hence might reduce the level of flea infestation, but might also result in the ingestion of Dipylidium infected adult fleas (Dryden and Rust, 1994). We investigated grooming behaviour to assess how often juveniles groom themselves and mothers groom their offspring and whether the frequency of grooming change with juvenile age. Overall we expected that grooming behaviour should increase the likelihood of infection of juvenile spotted hyaena by Dipylidium.
2. Material and methods
2.1. Study population
The study was conducted between 2003 and 2012 on three large, closely monitored clans that are part of a long-term research programme in the Serengeti National Park, Tanzania. Clans contained a mean ± SEM of 89 ± 1.49 animals, including a mean of 33 ± 5.8 adult females during the study period and approximately the same number of reproductively active adult males. All animals were individually known (East et al., 2003; Benhaiem et al., 2012) and their life histories were monitored (Hofer and East, 2003). The age of animals born in study clans was determined (±7 days) using pelage and other characteristics (Pournelle, 1965; East et al., 1989). Animals were considered adult when 24 months of age (East et al., 2009). Juveniles were categorised as ‘younger’ when <6 months of age (mean age 122.3 ± 3.7 days) and ‘older’ when between 6 months and 24 months of age (mean age 284.7 ± 15.4 days). The sex of juveniles was determined at approximately 3 months of age as detailed by Frank et al. (1990). Females reproduce throughout the year; their litters of one or two offspring (rare triplet litters never survive more than a few weeks in our study clans) are entirely dependent on maternal milk for their first six months of life (Hofer and East, 2003, 2008) and are weaned at between 12 and 18 months of age (Hofer and East, 1995). Younger juveniles rested during most daylight hours in underground burrows of communal dens. Older juveniles use underground burrows at the communal den but as they develop towards adulthood they increasingly rest elsewhere during the day. The underground burrows of communal dens were not accessible to adults (Hofer and East, 2008). Communal dens of all study clans were monitored during regular observations periods of several hours at dusk and dawn (Hofer and East, 1993a, Benhaiem et al., 2012).
Clan social structure consists of separate linear dominance hierarchies among adult females and reproductively active, mostly immigrant adult males (East and Hofer, 2001; East et al., 2003). The social rank of adult females and reproductively active males in their respective hierarchies was assessed using submissive responses in dyadic interactions (see East and Hofer, 2001; East et al., 2003). To compare rank positions across clans, individuals were assigned a standardised rank within a dominance hierarchy by distributing ranks evenly between the highest rank (standardised rank +1) and the lowest rank (standardised rank −1), with the median rank being scored as 0 (Goymann et al., 2001). Juveniles were assigned the rank of their mother (Hofer and East, 2003). All adult females and their offspring were socially dominant over adult immigrant males (East and Hofer, 2001; East et al., 2009).
Clan territories experienced large fluctuations in the abundance of migratory herbivores throughout the year (Hofer and East, 1993a,b). Prey abundance during observation periods in each clan territory was assigned to one of three categories as described by Hofer and East (1993a): (1) low (∼7.3 animals km−2) when only resident prey were present and all migratory prey were absent; (2) medium (∼31 animals km−2) when resident prey plus scattered herds of migratory prey were present; and (3) high (∼238 animals km−2) when resident prey and numerous, large herds of migratory prey were present. Prey abundance profoundly affected the distances at which adult clan members foraged from the clan communal den and thus influenced a mother’s absence interval (in number of days) from the den (Hofer and East, 1993b,c) and how frequently she nursed her offspring (Hofer and East, 1993c, 2008). When prey abundance was high, all lactating females nursed their litters daily; when low, all undertook commuting trips (Hofer and East, 1993b,c).
To examine whether self-grooming by juveniles changed with age we observed 47 juveniles (21 younger and 26 older juveniles) during one hour observation periods conducted at dawn or dusk and scored whether juveniles groomed themselves or not. When self-grooming occurred the animal was scored as 1, and no self-grooming was scored as 0. For animals observed during more than one observation period a mean score was calculated. For example, if grooming occurred in 2 of 4 observation periods this was scored as 0.5. To assess how often mothers groomed juveniles younger than 6 months and juveniles older than 6 months when present at the den, we also scored, in a similar manner, whether mothers groomed their offspring.
2.2. Sample collection for genetic screening and identification of Dipylidium
In total, 146 faecal samples were collected from 124 spotted hyaenas aged between 48 days and 12.7 years (117 juveniles, 20 adults). To compare infection in the same individual when juvenile and again when adult, 13 animals were sampled when juvenile and again when adult (mean time between adult and juveniles samples 3.3 years ± 186.8 days). All faeces were collected immediately after deposition, typically from communal latrines in the vicinity (8–100 m) of the communal den. The presence or absence of Dipylidium proglottids on faeces (Fig. 1c) was systematically noted for 117 samples when collected.
Faecal samples obtained shortly after deposition were mixed, sub-sampled and preserved in RNAlater (Sigma–Aldrich Inc., St Louis, MO, USA), and initially stored frozen at −10 °C, transported frozen and then stored at −80 °C. Gravid proglottids were collected from faeces and preserved in either 70% ethanol or RNAlater for genetic analysis. Proglottids in RNAlater were stored frozen.
To compare sequence data from Dipylidium sp. obtained from spotted hyaenas in Tanzania to sequence data for the same gene fragment of D. caninum in GenBank we extracted total DNA from proglottids using a NucleoSpin Soil kit (Macherey–Nagel, Düren, Germany) following the manufacturer’s instructions. This procedure included a homogenisation step (Precellys 24, Bertin Technologies, Montigny-le-Bretonneux, France) to break cestode cells. DNA concentration and quality were assessed using a NanoDrop ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). Extracts were stored at −20 °C.
Initially we used published sequences for cestode primer pairs (60.for. and 375.rev.) and a published protocol described by von Nickisch-Rosenegk et al. (1999) to obtain a conserved, short fragment of 314 base pair (bp) (minus primers) of the mitochondrial 12S rRNA gene. Polymerase chain reactions (PCRs) were performed with approximately 20–40 ng template DNA in 50 μl reactions containing 6.25 μM of each primer, 1.25 units Maxima Hot Start PCR Mastermix (Fermentas GmbH, St. Leon-Rot, Germany) and nuclease free water. The PCR program comprised an initial denaturation step of 4 min at 95 °C, 35 cycles at 94 °C for 60 s, 55 °C for 45 s and 55 °C for 45 s, followed by a final extension step at 72 °C for 4 min. We then used the sequence data we generated for this short fragment plus recently published sequence data of the complete mitochondrial gene from D. caninum (Nakao et al., 2013, GenBank Accession No. AB732959.1) to design two new primer pairs (Dca_rrnL1 – 4) to obtain a longer fragment that included the complete mitochondrial 12S rRNA gene plus a large section of the 16S rRNA gene. The PCR was performed in a 25 μl volume with 50 pM of each primer (Dca_rrnL1 5′ TGA GTT AAG ACC GGC GTG AG 3′ and Dca_rrnL2 5′ TTG ACA CCT TCC CCT GAA CG 3′; Dca_rrnL3 5′ TGG TAG TGC CTG CTC TAT GTT 3′ and Dca_rrnL4 5′ TAA GAA CCG ACC TGG CTC AC 3′) and 0.75 units Maxima Hot Start PCR Mastermix. After an initial denaturation step for 4 min at 95 °C, amplification was carried out for 35 cycles as follows: denaturation for 30 s at 95 °C, annealing for 45 s at 55 °C and elongation for 1 min at 72 °C. We sequenced two overlapping fragments of 542 and 447 bp respectively. We combined sequence data from all three fragments to obtain a fragment of 1176 bp including gaps but excluding primers.
Faecal samples were screened using the same DNA extraction procedure we applied to proglottids. For screening we designed primers 68.for (5′-AGC AAG TGA ATC CGT TCA-3′) and 236.rev (5′-GCA TCA AAA CTC TAA TAA GCA GCA C-3′) targeting a conserved 169 bp fragment of the mitochondrial 12S rRNA gene of D. caninum using Primer3 v.0.4.0 (Rozen and Skaletsky, 2000). Primer specificity was evaluated by in silico validation using nucleotide BLAST. There was no cross-reaction with host DNA. Correct amplification of the target fragments was confirmed by sequencing three randomly selected PCR products. DNA extracts were screened for Dipylidium in 10 μl PCRs consisting of 10–15 ng template DNA, 2.5 μM of each primer, 0.25 units DreamTaq™ PCR MasterMix (2X) (Fermentas GmbH, St. Leon-Rot, Germany) and nuclease-free water. PCR cycling conditions started with initial denaturation at 95 °C for 3 min, followed by 35 cycles at 94 °C for 30 s, 57 °C for 30 s, 72 °C for 45 s, and terminated with a final elongation step at 72 °C for 4 min. We investigated PCR sensitivity using a serial dilution of Dipylidium DNA extract mixed within faeces from adult captive spotted hyaenas not infected with any species of Dipylidium or other cestode species, at a volume of 1:1. We detected PCR products at all the following dilutions of 0.25, 0.125, 0.025, 0.0125 and 0.005 DNA/μl.
PCR products were either purified using RapidTip (Diffinity Genomics, West Henrietta, NY, USA) or QIA quick Gel Extraction (Qiagen GmbH, Hilden, Germany). Sequencing was bidirectional and conducted using a BigDye® Terminator Cycle sequencing kit 3.1(Applied Biosystems [ABI], Darmstadt, Germany). PCR products were analysed by gel electrophoresis and visualised by GelRed (Biotium Inc, Hayward, CA, USA) staining. Sequences were visualised on a Hitachi 3130 Genetic Analyzer (ABI) and identified using BLAST search in GenBank database (Altschul et al., 1997). Nucleic acid sequences were aligned using ClustalW (Larkin et al., 2007) in MEGA 5.05 (Tamura et al., 2011). Information on the presence or absence of Dipylidium proglottids observed on faeces was not known when samples were screened.
2.3. Measurement of faecal glucocorticoid metabolite concentrations (fGMCs)
We measured fGMCs in 53 faecal samples from 51 juvenile spotted hyaenas, including 50 samples also screened for infection by Dipylidium species and faeces from three juveniles obtained within a short period (⩽6 days) following sampling to screen for infection. Faeces were collected immediately after deposition, mechanically mixed, sub-sampled and stored as described by Benhaiem et al. (2012). fGMCs were quantified using a cortisol-3-CMO enzyme immunoassay validated for spotted hyaenas (Benhaiem et al., 2012). fGMCs were measured as ng/g faecal matter. Intra-assay and inter-assay coefficients of variation were 16.5% and 23.1% for a low (N = 9), and 5.3% and 10.1% for a high concentration pool (N = 11) (Benhaiem et al., 2012).
2.4. Statistical analysis
Statistical analyses were undertaken using SYSTAT 13.0 (Systat Software Inc., Richmond, VA, USA). Means are given ±SEM. To avoid pseudo-replication in the analyses we used only one (randomly chosen) faecal sample per juvenile. We used a Fisher’s exact test to compare the prevalence of Dipylidium sp. positive animals in 117 juveniles (including 52 females and 65 males) and 20 adults. A Wilcoxon signed-rank test was used to compare infection in 13 females screened when juvenile and again later as adults. We applied a Chi-square test to compare the incidence of positive samples in faeces with fGMCs above or below the mean concentration in juveniles. We used binary logistic regression models to investigate biologically relevant factors that might influence infection likelihood in juveniles, including those likely to alter flea infestation at communal dens and juvenile immune status. Infection of an animal (the dependent variable) was scored as present if the result of PCR screening was positive or Dipylidium proglottids were present on the faeces when collected, or absent if the PCR screening result was negative and no proglottids were observed on the faeces when collected. Our final model (Table 2) included the following parameters for the date on which each faecal sample was collected: (1) the number of younger juveniles present at the communal den, the most naïve age class to Dipylidium infection; (2) the number of adults and older juveniles present at the communal den as an index of the number of adult fleas brought to the den and of the flea eggs these fleas were likely to shed at the den; (3) maternal standardised rank as an index of juvenile body resources; (4) prey abundance (3 levels: high, medium and low) as an index of current maternal input in terms of the interval between visits to nurse offspring at the den; and (5) juvenile age in days. This was the model with the smallest values for the Akaike Information Criterion (AIC) and Schwarz’s Bayesian Information Criterion (BIC). In preliminary models we had considered alternative indices of the number of adult fleas brought to the den, namely the number of adult males at the den, the number of adult females at the den and total clan size. Replacing these parameters with parameter (2) above substantially reduced the AIC and BIC. We also considered clan identity as a possible source of variation and fGMCs as a measure of the allostatic load of each juvenile (Benhaiem et al., 2013). Removing these from the preliminary model also reduced the AIC and BIC. Models were considered equally parsimonious when the differences in AIC and BIC were less than 2. The significance threshold of tests was fixed at 5% and all tests were two-tailed.
Table 2.
The influence of ecological and social factors on the likelihood of infection with Dipylidium sp. in juvenile spotted hyaenas. Results show the estimates of the parameters, their standard errors and their lower and upper 95% confidence intervals, and the z-value with associated p-values from a binary logistic regression. Negative parameter estimates indicate that an increase in the value of the parameter reduced the likelihood of infection. Positive parameter estimates indicate that an increase in the value of the parameter enhances the likelihood of infection. This model was selected using Akaike Information Criterion and Schwarz’s Bayesian Information Criterion values.
| Parameter | Estimate | Standard error | Z | p-Value | 95% confidence intervals |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Constant | 2.251 | 1.203 | 1.872 | 0.061 | −0.106 | 4.609 |
| Total number of younger juveniles | −0.188 | 0.069 | −2.273 | 0.007 | −0.323 | −0.053 |
| Maternal standardised rank | 0.497 | 0.407 | 1.221 | 0.222 | −0.301 | 1.295 |
| Number of adults and older juveniles | 0.129 | 0.039 | 3.336 | 0.0009 | 0.053 | 0.206 |
| High prey abundance | −0.317 | 0.687 | −0.462 | 0.644 | −1.663 | 1.028 |
| Low prey abundance | −1.285 | 0.545 | −2.357 | 0.018 | −2.354 | −0.216 |
| Age | −0.003 | 0.002 | −1.681 | 0.093 | −0.008 | 0.0006 |
3. Results
3.1. Characterisation of the Dipylidium species infecting spotted hyaena
Eggs contained in packets obtained from proglottids were morphologically indistinguishable from those of D. caninum (Fig. 1b). We obtained six identical sequences of 314 bp in length from the mitochondrial 12S rRNA gene, using gravid proglottids obtained from the faeces of six different spotted hyaenas and used one of these samples to obtain the longer fragment (1176 bp). Comparison of sequence data from our shorter fragment with the two published D. caninum sequences of the same fragment revealed a high (99%) similarity to one sequence from Europe (accession number L49460.1) but a considerably lower similarity (89%) to one sequence from Asia (accession number AB031362.1). Comparison of sequence data from our longer fragment (accession number KF202097) with the only similar fragment from D. caninum also revealed a relatively low similarity (89%).
3.2. Comparison of visible evidence of infection and PCR screening results
Of the total 146 samples screened, 89 (61%) were positive by PCR and/or the presence of proglottids. Of 117 samples for which we had both a PCR screening result and visual score for the presence or absence of proglottids, 66 (56.4%) were positive by PCR and 57 (48.7%) had at least one proglottid visible at collection. Overall, PCR screening results produced a higher (by 7.7%) prevalence of infection than the visual scoring of proglottid presence but there was no significant difference between these methods in the probability of detecting infection (Chi-squared test, χ2 = 1.39, df = 1, p = 0.24). Even so, both methods apparently yielded false negative results. Dipylidium proglottids were observed on the majority (68.2%, 45/66 samples) of faeces identified as positive by PCR screening, indicating false negative results in 31.8% of samples. Of 51 faeces identified as negative by PCR screening, proglottids were observed on 12 samples (23.5%), indicating false negative PCR results.
3.3. Infection prevalence: the effect of age
Juveniles were significantly more often infected than adults (55.1% and 15.8%, respectively; Fisher’s exact test, p < 0.002), but prevalence of infection between younger (63.3%) and older juveniles (74.1%) did not differ (Fisher’s exact test, p = 0.15). A within-individual comparison of the impact of age on infection in 13 females screened when juvenile and when adult also revealed that these females were more often infected when juvenile than when adult (Wilcoxon signed-rank test, W = 21, exact p = 0.031). Of these 13 females, 8 (61%) were infected when juvenile and only 2 (14.4%) when adult. Infection among 52 juvenile females and 65 juvenile males was not influenced by sex (Fisher’s exact test, p = 0.70).
3.4. Faecal glucocorticoid metabolite concentrations (fGMCs) in juveniles
Juvenile fGMCs ranged between 13.1 and 395.1 ng/g (mean 51.0 ± 10.9 ng/g). Few juveniles had fGMCs that could be considered significantly elevated as only 8 juveniles had concentrations above the mean. The prevalence of infection in samples with fGMCs below (27 of 45) or above (6 of 8) the mean was similar (60% and 75%, respectively; Chi-squared test, χ2 = 0.65, df = 1, p = 0.42).
3.5. Factors modulating Dipylidium infection in juveniles
The results of the binary logistic regression (log likelihood ratio test, G = 72.24, df = 6, p = 0.00002, Table 2) revealed that the likelihood of infection in juveniles significantly decreased as the number of younger juveniles in the clan increased whereas the likelihood of infection increased with the combined number of adults and older juveniles that visited the den (means: 7.26 ± 0.34 adult females; 2.42 ± 0.21 adult males; 6.5 ± 0.27 older juveniles). In contrast to our prediction, the likelihood of infection was significantly lower during periods of low prey abundance in the clan territory than in periods with medium or high prey abundance (Fig. 2). Inclusion of juvenile age decreased both the AIC and Schwarz’s BIC values but age did not have a significant effect (Table 2). Infection was not influenced by maternal standardised rank. Inclusion of fGMCs, clan identity or clan size in the model decreased its significance and increased its AIC and Schwarz’s BIC values, resulting in weaker models than the results for that presented in Table 2.
Fig. 2.
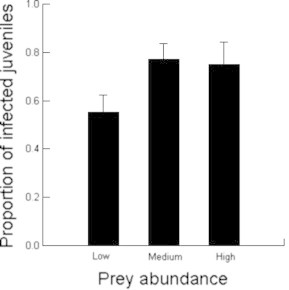
The effect of prey abundance in spotted hyaena clan territories on the proportion of juveniles infected with Dipylidium sp.
3.6. Grooming behaviour
There was a non-significant trend for older juveniles to have higher grooming scores, i.e. to groom themselves more often (0.51 ± 0.08, N = 27) than younger juveniles (0.27 ± 0.87, N = 21, Mann–Whiney U Test, U = 202.0, df = 1, p = 0.067). Mothers were equally likely to groom younger and older offspring when they visited the den (maternal grooming scores: 0.51 ± 0.11, N = 16 younger juveniles; 0.60 ± 0.11, N = 20 older juveniles, U = 141.0, df = 1, p = 0.51).
4. Discussion
Nucleotide sequence data of a relatively short fragment of the mitochondrial 12S rRNA gene from the Dipylidium sp. infecting spotted hyaena in the Serengeti National Park revealed a high similarity to the same short gene fragment described from D. caninum in Europe, but a lower similarity to D. caninum in Asia. Comparison of our longer fragment with the one other similar fragment available from D. caninum of unknown geographical origin (Nakao et al., 2013) revealed a relatively low similarity (89%). This genetic comparison is not sufficient to establish whether the Dipylidium species described to infect spotted hyaena hosts is D. caninum or a closely related species.
Our investigation of factors influencing the prevalence of Dipylidium infection in spotted hyaenas, which combined visible evidence of infection with results from genetic screening, revealed that juveniles were more often infected than adults. Among juveniles, the likelihood of infection decreased with the number of younger juveniles at the den and also during periods of low prey abundance in clan territories. The likelihood of infection increased as the combined total number of adults and older juveniles visiting the den increased.
Our results revealed that the likelihood of Dipylidium infection in juvenile spotted hyaenas was modulated by several factors. The decline in infection in individuals from when they were juvenile to when they were adult suggests that most animals in the population had acquired immunity against Dipylidium infection by the time they reached adulthood. This suggests an increased development of an immunological memory of Dipylidium with age. It is worth noting that although mothers frequently groomed their offspring, and presumably often ingested Dipylidium infected fleas, only a relatively small proportion of adult females were infected with Dipylidium. This suggests that effective immunity against Dipylidium infection in the adult population may be maintained by the repeated ingestion of infected fleas throughout adulthood. A similar decrease in the prevalence of coronavirus infection with increasing age was observed in our study population (Goller et al., 2013) with juveniles having lower coronavirus antibody titres than adults (East et al., 2004). Another possible reason for the decreased prevalence of Dipylidium infection in adults is the development of cross immunity following infection with other cestode species. Ungulates are intermediate hosts of cestodes whose final hosts are carnivores. Decreased reliance on maternal milk during weaning is highly likely to lead to infection with other cestodes during adulthood which may cause the elimination of Dipylidium.
Our results indicate that the maintenance of Dipylidium infection in spotted hyaena clans requires the regular addition of susceptible juveniles to clan communal dens. Clans in the Serengeti National Park are large, and during this study contained a mean of 89 individuals, including approximately 33 adult females per clan. These females produced naïve young throughout the year, thereby regularly introducing new clan members susceptible to Dipylidium infection. Once infected, juveniles maintain infection in the flea population at communal dens in each study clan. Moreover, we interpret the significant increase in the prevalence of Dipylidium infection in juveniles associated with an increase in the combined number of adults and older juveniles at communal dens (Table 2), to imply an increase in the size of the intermediate host (flea) population at these dens.
Interestingly, infection among juveniles decreased as the number of younger juveniles in a den increased (Table 2). This result may be caused by a reduced chance of an infected flea being ingested by a naïve (younger) juvenile, because of a decline in the number of infected fleas per younger juvenile as the number of younger juveniles increased (i.e., a dilution effect), coupled with the tendency of younger juveniles to groom themselves less often than older juveniles. If the main intermediate host of Dipylidium sp. infecting spotted hyaenas is a stick fast flea species, then it may also be that younger juveniles are less able to remove small fleas anchored to their skin than are older juveniles.
We expected that hungry juveniles that are infrequently nursed by their mother during periods of low prey abundance would trade-off immunity against costly functions such as maintenance or growth, and thus be more prone to Dipylidium infection. On the contrary, our model indicated that the prevalence of Dipylidium infection in juveniles significantly decreased during periods of low prey abundance (Table 2, Fig. 2) when juveniles often receive no milk from their mothers for several days (Hofer and East, 1993c, 2003, 2008). Three processes may explain this result. Firstly, because all adult clan members forage on distant, large concentrations of migratory herbivores when prey abundance in their territory is low, the low number of clan members visiting communal dens during periods of low prey abundance may cause a reduction in the flea population at the den. Secondly, during periods when juveniles receive no milk for several days, the nutrients available to Dipylidium (in the alimentary canal of hungry juveniles) for egg and proglottid production will be reduced. If the fecundity of Dipylidium is curtailed by decreased milk intake by juveniles, this might reduce infection prevalence in the intermediate (flea) population at the communal den. Finally, this result might simply reflect an increased number of false negative results caused by a low fecundity among adult Dipylidium in hungry juveniles. Interestingly, even though the offspring of high-ranking mothers are significantly better nourished than those of low-ranking mothers in the long-term (Hofer and East, 2003), there was no evidence that their offspring had a lower prevalence of infection.
We found no evidence that the likelihood of Dipylidium infection in juveniles was significantly altered by their fGMCs. However, we determined fGMCs in only 51 of the 117 juveniles in our study. This reduced the power of our model, and few of these juveniles had fGMCs likely to cause a biologically meaningful reduction in immune function. Furthermore, longer-term measures of fGMCs are required to identify chronically stressed juveniles in which immunosuppression might occur. As we determined only the presence or absence of Dipylidium infection, our results do not exclude the possibility that elevated fGMCs increase infection intensity in juveniles.
Juvenile spotted hyaenas less than two months of age rarely venture from the immediate vicinity of entrances to underground burrows of the communal den, and at this age their mothers typically consume their faeces. Older clan members at dens generally leave the den area and defaecate in communal latrines (East et al., 2004) which are at least eight metres from the den area. These two behaviours possibly decrease the chance of flea larvae in communal den areas consuming Dipylidium eggs voided in faeces. Even though the use of latrines most likely greatly curtails the number of Dipylidium eggs available for consumption by flea larvae in the den, proglottids or egg sacs that adhere to fur around the anus of infected animals may be transported to communal dens and infect fleas.
Studies of intestinal parasite infection in mammals apply either necropsy or coprology methods (Table 1). Necropsy requires either the opportunistic collection of carcasses, which may not provide a representative sample of a host population, or the sacrifice of animals. Coprology methods can provide a representative sample of a host population and also permit the repeated assessment of infection in the same animal, for example at different life history stages or during different ecological conditions. Even so, coprology methods can yield false negative results, regardless of whether traditional methods (i.e. egg flotation and identification) or genetic screening techniques are applied (e.g. Drogemuller et al., 2004) because of the prepatent period following initial infection and the intermittent release of eggs by intestinal parasites. Our assessment of our genetic screening method indicated that it was reasonably sensitive, but even so a proportion of negative faeces (23.5%) had Dipylidium proglottids on them when collected. We suggest these false negative genetic screening results probably were caused by the clumped distribution of Dipylidium DNA in faeces (i.e. eggs in packets and proglottids). We therefore suggest to combine data on the occurrence of proglottids on freshly deposited faeces with genetic screening results to reduce the chance of false negative results in studies of Dipylidium infection in mammalian hosts.
Within the diverse guild of wild carnivore species present in the Serengeti National Park, evidence of Dipylidium infection was reported in golden jackal (Canis aureus), side-striped jackal (Canis adustus) and silver-backed jackal (Canis mesomelas) in Kenya (Nelson et al., 1965, Table 1), and it is likely that these species are infected with Dipylidium in the Serengeti National Park. Studies on African lion (Panthera leo) in the Serengeti National Park did not find evidence of Dipylidium infection (Müller-Graf, 1995; Bjork et al., 2000). Coprology studies of Dipylidium infection in wild carnivores rarely describe the age of the animals from which samples were collected. Our results indicate that information on host age is essential for the interpretation of results and comparisons of infection prevalence across species.
Horak et al. (2004) reported that E. larina frequently infests the warthog (Phacochoerus aethiopicus) but normally not carnivores which may be because most carnivores do not share underground burrows with warthogs. However, spotted hyaenas use warthog burrows to rest during the day and occasionally warthogs use burrows within spotted hyaena denning area. If E. larina is the main intermediate host of Dipylidium in the Serengeti National Park, co-habitation of warthog burrows may have a positive effect of E. larina populations in spotted hyaena clans.
5. Conclusion
This study revealed key factors that influenced Dipylidium infection prevalence in a highly social, wild carnivore. It is the first to show decreased infection during periods of low prey abundance in a migratory ecosystem where carnivores experience large fluctuations in prey abundance in their territories. Our study highlights the importance of communal denning, the presence of susceptible juveniles and the intermediate host population at dens for Dipylidium infection in spotted hyaena clans. We suspect that host age and denning behaviour are important factors influencing Dipylidium infection prevalence in other wild carnivores. More genetic information is needed from D. caninum worldwide to clarify whether Dipylidium species that infect wild carnivores are D. caninum or distinct species.
Acknowledgements
We are grateful to the Tanzania Commission of Science and Technology, the Tanzania Wildlife Research Institute, and the Tanzania National Parks for permission to conduct this study, and the Tierpark Berlin for providing faeces from their captive spotted hyaenas. We thank Malvina Andris, Nelly Boyer, Janine Helms and Dagmar Thierer for their assistance, Jürgen Krücken, Janina Demeler and Markus von Nickisch-Rosenegk for helpful advice regarding cestode identification. We thank the editor and two referees for their helpful comments. This work was financed by a grant awarded from the Leibniz Competitive Fund (“Pakt für Forschung und Innovation”) made possible by the Federal German government through its Ministry for Education and Research and the community of German states (“Länder”), and the Leibniz Institute for Zoo and Wildlife Research.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J.H., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutzki D., Schaper R. Endoparasites in dogs and cats in Germany 1999–2002. Parasitol. Res. 2003;90:148–150. doi: 10.1007/s00436-003-0922-6. [DOI] [PubMed] [Google Scholar]
- Benhaiem S., Dehnhard M., Bonanni R., Hofer H., Goymann W., Eulenberger K., East M.L. Validation of an enzyme immunoassay for the measurement of faecal glucocorticoid metabolites in spotted hyenas (Crocuta crocuta) Gen. Comp. Endocrinol. 2012;178:265–271. doi: 10.1016/j.ygcen.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Benhaiem S., Hofer H., Dehnhard M., Helms J., East M.L. Sibling competition and hunger increase allostatic load in spotted hyaenas. Biol. Lett. 2013;9:20130040. doi: 10.1098/rsbl.2013.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitam I., Dittmar K., Parola P., Whiting M.F., Raoult D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010;14:e667–e676. doi: 10.1016/j.ijid.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Bjork K.E., Averbeck G.A., Stromberg B.E. Parasites and parasite stages of free-ranging wild lions (Panthera leo) of Northern Tanzania. J. Zoo Wildl. Med. 2000;31:56–61. doi: 10.1638/1042-7260(2000)031[0056:PAPSOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Calvete C., Lucientes J., Castillo J.A., Estrada R., Gracia M.J., Peribáñez M.A., Ferrer M. Gastrointestinal helminth parasites in stray cats from the mid-Ebro valley, Spain. Vet. Parasitol. 1998;75:235–240. doi: 10.1016/s0304-4017(97)00182-9. [DOI] [PubMed] [Google Scholar]
- Coop R.L., Kyriazakis I. Nutrition–parasite interaction. Vet. Parasitol. 1999;84:187–204. doi: 10.1016/s0304-4017(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Dalimi A., Sattari A., Motamedi G. A study of intertinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet. Parasitol. 2006;142:129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Drogemuller M., Beelitz P., Pfister K., Schnieder T., von Samson-Himmelstjerna G. Amplification of ribosomal DNA of Anoplocephalidae: Anoplocephala perfoliata diagnosis by PCR as a possible alternative to coprological methods. Vet. Parasitol. 2004;124:205–215. doi: 10.1016/j.vetpar.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Dryden M.W., Rust M.K. The cat flea: biology, ecology and control. Vet. Parasitol. 1994;52:1–19. doi: 10.1016/0304-4017(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Dubná S., Langrová I., Nápravík J., Vadlejch J., Pekár S., Fechtner J. The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Vet. Parasitol. 2007;145:120–128. doi: 10.1016/j.vetpar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- East M.L., Hofer H. Loud calling in a female-dominated mammalian society: II. Behavioural contexts and functions of whooping of spotted hyaenas, Crocuta crocuta. Anim. Behav. 1991;42:651–669. [Google Scholar]
- East M.L., Hofer H. Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav. Ecol. 2001;12:558–568. [Google Scholar]
- East M.L., Hofer H. Crocuta crocuta spotted hyena. In: Kingdon J., Hoffmann M., editors. vol. V. Bloomsbury; London: 2013. pp. 273–281. (Mammals of Africa: Carnivores, Pangolins, Equids and Rhinoceroses). [Google Scholar]
- East M.L., Hofer H., Türk A. Functions of birth dens in spotted hyaenas (Crocuta crocuta) J. Zool. Lond. 1989;219:690–697. [Google Scholar]
- East M.L., Burke T., Wilhelm K., Greig C., Hofer H. Sexual conflicts in spotted hyenas: male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proc. R. Soc. B. 2003;270:1247–1254. doi: 10.1098/rspb.2003.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M.L., Moestl K., Benetka V., Pitra C., Höner O.P., Wachter B., Hofer H. Coronavirus infection of spotted hyenas in the Serengeti ecosystem. Vet. Microbiol. 2004;102:1–9. doi: 10.1016/j.vetmic.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M.L., Höner O.P., Wachter B., Wilhelm K., Burke T., Hofer H. Maternal effects on offspring social status in spotted hyenas. Behav. Ecol. 2009;20:478–483. [Google Scholar]
- East M.L., Gusset-Burgener N., Hofer H. Sex differences in olfactory behaviours reflect the importance of scent marking for social integration in adult females and competition between reproductively active males in the spotted hyenas. In: East M.L., Dehnhard M., editors. Chemical Signals in Vertebrates 12. Springer; New York: 2013. pp. 149–160. [Google Scholar]
- Eguia-Aguilar P., Cruz-Reyes A., Maninez-Maya J.J. Ecological analysis and description of the intestinal helminths present in dogs in Mexico city. Vet. Parasitol. 2005;127:139–146. doi: 10.1016/j.vetpar.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Engh A.L., Nelson K.G., Peebles R., Hernandez A.D., Hubbard K.K., Holekamp K.E. Coprologic survey of parasites of spotted hyenas (Crocuta crocuta) in the Masai Mara National Reserve, Kenya. J. Wildl. Dis. 2003;39:224–227. doi: 10.7589/0090-3558-39.1.224. [DOI] [PubMed] [Google Scholar]
- Fontanarrossa M.F., Vezzani D., Basabe J., Eiras D.F. An epidemiological study of gastrointestinal parasites of dogs from Southern Greater Buenos Aires (Argentina): age, gender, breed, mixed infections, and seasonal and spacial patterns. Vet. Parasitol. 2006;136:283–295. doi: 10.1016/j.vetpar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Frank L.G. Social organisation of spotted hyena (Crocuta crocuta). I. Demography. Anim. Behav. 1986;34:1500–1509. [Google Scholar]
- Frank L.G. Social organisation of spotted hyena (Crocuta crocuta). II. Dominance and reproduction. Anim. Behav. 1986;34:1510–1527. [Google Scholar]
- Frank L.G., Glickman S.E., Powch I. Sexual dimorphism in the spotted hyaena (Crocuta crocuta) J. Zool. Lond. 1990;221:308–313. [Google Scholar]
- Gabriele F., Wakelin D., Palmas C. Specific cross immunity between Hymenolepis nana and H.iDiminuta: immunization with heterologous and homologous light infections. J. Helminthol. 1988;62:115–123. doi: 10.1017/s0022149x00011342. [DOI] [PubMed] [Google Scholar]
- Goller K.V., Fickel J., Hofer H., Beier S., East M.L. Coronavirus genotype diversity and prevalence of infection in wild carnivores in the Serengeti National Park, Tanzania. Arch. Virol. 2013;158:729–734. doi: 10.1007/s00705-012-1562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W., East M.L., Wachter B., Höner O., Möstl E., Van’t Hof T.J., Hofer H. Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyaenas. Proc. R. Soc. Lond. B Biol. Sci. 2001;268:2453–2459. doi: 10.1098/rspb.2001.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W., Wingfield J.C. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 2004;67:591–602. [Google Scholar]
- Hackett F., Walters T.M.H. Helminths of the red fox in mid-Wales. Vet. Parasitol. 1980;7:181–184. [Google Scholar]
- Hofer H., East M.L. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. I. Social organization. Anim. Behav. 1993;46:547–557. [Google Scholar]
- Hofer H., East M.L. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. II. Intrusion pressure and commuters’ space use. Anim. Behav. 1993;46:559–574. [Google Scholar]
- Hofer H., East M.L. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. III. Attendance and maternal care. Anim. Behav. 1993;46:575–589. [Google Scholar]
- Hofer H., East M.L. Population dynamics, population size, and the commuting system of Serengeti spotted hyaenas. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. The University of Chicago Press; Chicago: 1995. pp. 332–363. [Google Scholar]
- Hofer H., East M.L. Behavioural processes and costs of co-existence in female spotted hyenas: a life history perspective. Evol. Ecol. 2003;17:315–331. [Google Scholar]
- Hofer H., East M.L. Siblicide in Serengeti spotted hyenas: a long-term study of maternal input and cub survival. Behav. Ecol. Sociobiol. 2008;62:341–351. [Google Scholar]
- Hofer H., East M.L. Stress and immunosuppression as factors in the decline and extinction of wildlife populations: concepts, evidence and challenges. In: Aguirre A.A., Ostfeld R.S., Daszak P., editors. New Directions in Conservation Medicine: Applied Cases of Ecological Health. Oxford University Press; New York: 2012. pp. 82–107. [Google Scholar]
- Holekamp K.E., Smale L., Szykman M. Rank and reproduction in the female spotted hyena. J. Reprod. Fertil. 1996;108:229–237. doi: 10.1530/jrf.0.1080229. [DOI] [PubMed] [Google Scholar]
- Horak I.G., Beaucournu J.-C., Braack L.E.O. Parasites of domestic and wild animals in South Africa. XLIV. Fleas (Insecta: Siphonatera: Pulicidae) collected from 15 carnivore species. Onderstepoort J. Vet. Res. 2004;71:9–14. doi: 10.4102/ojvr.v71i1.281. [DOI] [PubMed] [Google Scholar]
- Ito A., Onitake K., Andreassen J. Lumen phase specific cross immunity between Hymenolepis microstoma and H. nana in mice. Int. J. Parasitol. 1988;18:1019–1027. doi: 10.1016/0020-7519(88)90071-9. [DOI] [PubMed] [Google Scholar]
- Khalafalla R.E. A survey study on gastrointestinal parasites of stray cats in northern region of Nile delta, Egypt. PLoS One. 2011;6:e20283. doi: 10.1371/journal.pone.0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk H. Chicago University Press; Chicago: 1972. The Spotted Hyena. [Google Scholar]
- Labarthe N., Serrão M.L., Ferreira A.M.R., Almeida N.K.O., Guerrero J. A survey of gastrointestinal helminths in cats of the metropolitan region of Rio de Janeiro, Brazil. Vet. Parasitol. 2004;123:133–139. doi: 10.1016/j.vetpar.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. ClustalW and ClustalX version 2. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lightowlers M.W., Gauci C.G., Chow C., Drew D.R., Gauci S.M., Heath D.D., Jackson D.C., Dadley-Moor D.L., Read A.J. Molecular and genetic characterization of the hosts-protective onchosphere antigens of taeniid cestode parasites. Int. J. Parasitol. 2003;33:1207–1217. doi: 10.1016/s0020-7519(03)00174-7. [DOI] [PubMed] [Google Scholar]
- Magi M., Macchioni F., Dell’Omodarme M., Prati M.C., Calderini P., Gabrielli S., Iori A., Cancrini G. Endoparasites of red fox (Vulpes vulpes) in Central Italy. J. Wildl. Dis. 2009;45:881–885. doi: 10.7589/0090-3558-45.3.881. [DOI] [PubMed] [Google Scholar]
- Martinez-Moreno F.J., Hernandez S., Lopez-Cobos E., Becerra C., Acosta I., Martinez-Moreno A. Estimation of canine intestinal parasites in Cordoba (Spain) and their risk to public health. Vet. Parasitol. 2007;143:7–13. doi: 10.1016/j.vetpar.2006.08.004. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Wingfield J.C. What’s in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010;57:105–111. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D.M. The immune response to and immunomodulation by Hymenolepis diminuta. Parasitology. 2010;137:385–394. doi: 10.1017/S0031182009990886. [DOI] [PubMed] [Google Scholar]
- Minnaar W.N., Krecek R.C., Fourie L.J. Helminths in dogs from a peri-urban resource-limited community in Free State Province, South Africa. Vet. Parasitol. 2002;107:343–349. doi: 10.1016/s0304-4017(02)00155-3. [DOI] [PubMed] [Google Scholar]
- Molina C.P., Ogburn J., Adegboyega P. Infection by Dipylidium caninum in an infant. Arch. Pathol. Lab. Med. 2003;127:e157–9. doi: 10.5858/2003-127-e157-IBDCIA. [DOI] [PubMed] [Google Scholar]
- Müller-Graf C.D.M. A coprological survey of intestinal parasites of wild lions (Panthera leo) in the Serengeti and Ngorongoro Crater, Tanzania, East Africa. J. Parasitol. 1995;81:812–814. [PubMed] [Google Scholar]
- Nakao M., Lavikainen A., Iwaki T., Haukisalmi V., Konyaev S., Oku Y., Okamoto M., Ito A. Molecular phylogeny of the genus Taenia (Cestoda: Taeniidae): Proposal for the reconstruction of Hydatigera Lamarck, 1916 and a creation of a, new genus Versteria. Int. J. Parasitol. 2013;43:427–437. doi: 10.1016/j.ijpara.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Nelson G.S., Pester F.R.N., Rickman R. The significance of wild animals in the transmission of cestodes of medical importance in Kenya. Trans. R. Soc. Trop. Med. Hyg. 1965;59:507–524. doi: 10.1016/0035-9203(65)90153-7. [DOI] [PubMed] [Google Scholar]
- Nichol S., Ball S.J., Snow K.R. Prevalence of intestinal parasites in feral cats in some urban areas of England. Vet. Parasitol. 1981;9:107–110. doi: 10.1016/0304-4017(81)90028-5. [DOI] [PubMed] [Google Scholar]
- Nonaka N., Nakamura S., Inoue T., Oku Y., Katakura K., Matsumoto J., Mathis A., Chembesofu M., Phiri I.G.K. Coprological survey of alimentary tract parasites in dogs from Zambia and evaluation of a coproantigen assay for canine echinococcosis. Ann. Trop. Med. Parasitol. 2011;105:521–530. doi: 10.1179/2047773211Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Sequeira T.C.G., Amarante A.F.T., Ferrari T.B., Nunes L.C. Prevalence of intestinal parasites in dogs from São Paulo State, Brazil. Vet. Parasitol. 2002;103:19–27. doi: 10.1016/s0304-4017(01)00575-1. [DOI] [PubMed] [Google Scholar]
- Palmer C.S., Thompson R.C.A., Traub R.J., Rees R., Robertson I.D. National study of the gastrointestinal parasited of dogs and cats in Australia. Vet. Parasitol. 2008;151:181–190. doi: 10.1016/j.vetpar.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Pournelle G.H. Observations on the birth and early development of the spotted hyaena. J. Mammal. 1965;46:503. [Google Scholar]
- Pugh R.E. The effect on the development of Dipylidium caninum on the hosts reaction to this parasite in the adult flea Ctenocephalides felis felis. Parasitol. Res. 1987;73:171–177. doi: 10.1007/BF00536475. [DOI] [PubMed] [Google Scholar]
- Richards D.T., Harris S., Lewis J.W. Epidemiological studies on intestinal helminth parasites of rual and urban foxes (Vulpes vulpes) in the United Kingdom. Vet. Parasitol. 1995;59:39–51. doi: 10.1016/0304-4017(94)00736-v. [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H.J. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S., Misener S., editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, USA: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Santos J.L., Magalhães N.B., dos Santos H.A., Ribeiro R.R., Guimarães M.P. Parasites of domestic and wild canids in the region of Serra do Cipó National Park, Brazil. Rev. Bras. Parasitol. Vet. 2012;21:270–277. doi: 10.1590/s1984-29612012000300016. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Oxford University Press; Oxford: 2011. Evolutionary Parasitology. The Integrated Study of Infections, Immunology, Ecology, and Genetics. [Google Scholar]
- Segovia J.M., Torres J., Miquel J., Llaneza L., Feliu C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001;75:183–192. [PubMed] [Google Scholar]
- Smale L., Frank L.G., Holekamp K.E. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Anim. Behav. 1993;46:467–477. [Google Scholar]
- Spinage C.A. Springer; New York: 2012. African Ecology. pp 1159. [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Nickisch-Rosenegk M., Lucius R., Loos-Frank B. Contributions to the phylogeny of the Cyclophyllidea (Cestoda) inferred from mitochondrial 12S rDNA. J. Mol. Evol. 1999;48:586–596. doi: 10.1007/pl00006501. [DOI] [PubMed] [Google Scholar]
- Wang A., McKay D.M. Immune modulation by a high molecular weight fraction from the rat tapeworm Hymenolepis diminuta. Parasitology. 2005;130:575–585. doi: 10.1017/s0031182004006985. [DOI] [PubMed] [Google Scholar]
- Wingfield J.C., Maney D.L., Breuner C.W., Jacobs J.D., Lynn S., Ramenofsky M., Richardson R.D. Ecological bases of hormone-behavior interactions: the emergency life-history stage. Am. Zool. 1998;38:191–206. [Google Scholar]
- Wong M.H. Multiple infections with Dipylidium caninum in an infant. Can. Med. Assoc. J. 1955;72:453–455. [PMC free article] [PubMed] [Google Scholar]


