Graphical abstract

Keywords: Alopochen aegyptiacus, Plasmodium nucleophilum, Brazil, Goose, Avian malaria diagnosis, P. (Haemamoeba) sp.
Highlights
-
•
An Egyptian Goose (Alopochen aegyptiacus) died in São Paulo Zoo, Brazil.
-
•
Plasmodium (Novyella) nucleophilum was identified, using microscopy and PCR.
-
•
The first assignment of the cytb gene sequence to P. nucleophilum is reported.
-
•
Phylogenetic position of P. nucleophilum was determined.
-
•
This sequence will assist in barcoding of this Plasmodium species in other birds.
Abstract
Plasmodium (Novyella) nucleophilum was identified using microscopy and PCR, in an Egyptian Goose (Alopochen aegyptiacus) that died in São Paulo Zoo, Brazil. This parasite is characterized by elongated gametocytes, small meronts with scant cytoplasm, less than eight merozoites and mainly for having all the stages appressed to the nuclei of infected erythrocytes. Additionally, Plasmodium (Haemamoeba) sp. was identified by microscopy in the same blood sample. The latter parasite lacks nucleophilic blood stages and is characterized by large roundish trophozoites, each with a large prominent centrally collated vacuole. This co-infection was not confirmed by PCR amplification of the mitochondrial cytochrome b (cytb) gene and sequencing; only one Plasmodium sp. cytb sequence was detected in the blood sample. Since parasitemia of P. nucleophilum (2.4%) was much higher than that of P. (Haemamoeba) sp. (0.2%), PCR may have favored the amplification of the cytb sequence of the former. Phylogenetic analysis is in agreement with this conclusion because the reported cytb sequence was positioned in the same branch of sequences of several Novyella species. This is the first assignment of the mitochondrial cytb gene sequence to P. nucleophilum. The P. (Haemamoeba) parasite is particularly similar to Plasmodium (Haemamoeba) tejerai, because its advanced trophozoites and young erythrocytic meronts possess a large vacuole with prominent pigment granules arranged around it, the characteristic features of development in this species. For definitive identification of P. (Haemamoeba) species, mature meronts and gametocytes are required; however, these were absent from the thin blood smear. Representative images of the blood stages of P. nucleophilum and P. (Haemamoeba) sp. are provided. Together with microscopy data, the P. nucleophilum cytb sequence will assist in molecular identification (barcoding) of this Plasmodium species in other birds.
1. Introduction
Haemosporidian parasites (Sporozoa: Haemosporida) are cosmopolitan protists that parasitize birds and other animals and use blood-sucking dipteran insects as vectors. More than 200 species of avian haemosporidians from hundreds of bird species have been described including over 50 morphologically distinct species of the genus Plasmodium belonging to five subgenera (Valkiūnas, 2005; Zehtindjiev et al., 2012). Brazil has one of the richest bird faunas on the planet and some species of captive and wild birds have been shown to be infected with blood protists, including parasites belonging to all subgenera of Plasmodium, but only a few studies have been conducted on biology of these parasites (rev. in Braga et al., 2011).
In zoological parks, the animals live in a high density, close to animals from different zoogeographical regions, which can favor parasite infections (Primarck and Rodrigues, 2001; Silva and Corrêa, 2006). Many zoos have rich vegetation and are located in bush lands, which are visited by migrant birds every year. Infected migrant birds are potential donors of parasitic infections, which can be transmitted to zoo birds, particularly Plasmodium infections, since the relationship between the parasite and host is of low specificity (Bensch et al., 2000). Thus, studies involving parasites of migrating birds and the zoo animals that are in proximity to them are important for wildlife conservation.
The Atlantic forest is considered as one of world’s biodiversity hot spots and some of the best and most extensive examples of this biome are in São Paulo (Ribeiro et al., 2009). São Paulo Zoo, established in 1958, is located in one of the last forest remnants within the City of São Paulo, the largest city in Brazil, with a high density and diversity of mosquitoes transmitting avian malaria (Guimarães et al., 2000; Bueno et al., 2010; Ribeiro et al., 2012). The zoo is located in an area of 824,529 m2 of original Atlantic Forest with the headwaters of the historical Ipiranga stream, whose waters form a lake that receives several wild species of birds including migratory species. Around the lake, the forest shelters wild native animals, forming diverse wildlife parallel to the zoo animal collection. More than 3000 animals are on display, representing species of mammals, birds, reptiles, amphibians and invertebrates. During migration and the wintering of birds from various American countries, as well as from other Brazilian regions, the space destined for zoo animals is shared by migratory species, including opportunistic species. The latter are behaviorally flexible birds living in variable environmental conditions that are sustained by different food sources. Thus, living freely in the area of the zoo, such birds can rapidly take advantage of favorable conditions when they arise. Because blood-sucking mosquitoes that can transmit avian malaria are widespread in the zoo (Bueno et al., 2010) and favorable abiotic conditions for malaria transmission also exist, we initiated an investigation in São Paulo Zoo in order to estimate the prevalence of Plasmodium spp. in birds using both PCR-based and microscopy methods. In this paper, we report a malaria infection in an Egyptian Goose (Alopochen aegyptiacus) that was born in São Paulo Zoo and lived in one of the lakes where migratory birds are present during their migration and wintering. Two Plasmodium species were identified and a mitochondrial cytochrome b (cytb) gene sequence was determined, enabling the molecular characterization of one these parasites. Illustrations of blood stages of both parasites are provided, and phylogenetic analysis identified DNA lineages closely related to them.
2. Material and methods
2.1. Case history
A 10-year-old adult male goose, born at the São Paulo Zoo, was referred to the Veterinary Division with clinical signs of incoordination, in October 2011. During clinical examination, it was apathetic, skinny, dehydrated and presented pale mucosa. The goose had a small injury in the cloacal region, wet feathers and a lot of dipteran larvae all over its body. It was physically restrained for blood sample collection, venous access to administration of fluid with glucose and antibiotics (enrofloxacin), larvae removal and cleaning and drying of the feathers. During the procedure, the goose had a cardiorespiratory arrest, reanimation was unsuccessful and it died. The blood examination indicated that it was anemic with hematocrit levels of 31% (reference values are 41–54%). It presented severe leukocytosis, 88 × 103 μL (6.39–21.73 × 103 μL), and heterophilia, 81.84 × 103 μL (2.62–13.41 × 103 μL), indicating the presence of an infectious agent. Blood biochemistry results were abnormal: uric acid and aspartate aminotransferase (AST) were very high, 28.5 mg/dL (3.0–8.6 mg/dL) and 385 UI/L (9–75 UI/L), respectively. The reference values (in parenthesis) were obtained from ISIS 2002 (http://www.isis.org). During necropsy, thoracic air sac presented with a yellowish color and fungal colonies, suggesting Aspergillus sp. infection, which was confirmed by histopathology. Plasmodium sp. parasitemia, splenomegaly, a severe pulmonary edema and the presence of signs of multifocal necrosis on the liver were also observed. However, the hepatitis may have been caused by the fungal toxin released by Aspergillus sp. and could have contributed to the goose’s death (Quist et al., 2007). The causa mortis was likely respiratory insufficiency.
2.2. Collection of blood samples and representative material
Venous blood was collected from median metatarsal vein of the goose during clinical examination for haemogram and biochemistry investigation. A thin blood smear was prepared without anticoagulant and the remaining blood was placed in a lithium heparin tube and frozen for DNA extraction. The thin blood smear was fixed by methanol, stained by Giemsa and 100 microscopic fields were examined under light microscopy (1000×) (Valkiūnas, 2005). The intensity of parasitemia was determined, as recommended by Godfrey et al. (1987). The voucher thin blood smears from the infected goose were deposited in the Clinical Laboratory of the Zoological Park Foundation, São Paulo, Brazil (accession numbers 26679A and 26679B); digital images of the parasites are also available in the Clinical Laboratory and in the Institute of Ecology, Nature Research Centre, Vilnius, Lithuania. The sample of gDNA from the Egyptian Goose (original field number is 7885) is deposited in the Clinical Laboratory of the Zoological Park Foundation, São Paulo, Brazil.
Morphology of Plasmodium nucleophilum from the Egyptian Goose was compared with the neohapantotype preparations of the same parasite from experimentally infected domestic canary Serinus canaria domestica (R. D. Manwell’s original strain, accession nos. 640, 641) in the Garnham Collection at the Natural History Museum, London.
2.3. Goose genomic DNA (gDNA) extraction, PCR amplification of Plasmodium sp. cytb fragments, sequencing and sequence data analysis
After thawing, the blood sample was centrifuged and 100–150 μl of red blood pellets were used for the DNA extraction. First, an initial lyse was performed with 1% saponin. Next, the pellet was washed twice in ultrapure water and submitted to the extraction protocol with the GFX™ Genomic Blood DNA Purification Kit (Amersham Biosciences, GE Healthcare), following the manufacturer’s instructions. The gDNA was eluted in 100 μl of ultrapure water and stored at −20 °C. A fragment of 1.1 kb (approximately 92% of the gene) from the mitochondrial cytochrome b gene (cytb) was amplified using a nested PCR, taking standard precautions to prevent cross-contamination of samples. The PCR reactions were conducted as previously described (Perkins and Schall, 2002) using primers DW2 and DW4 and 5 μl of gDNA in the first reaction and 1 μl aliquot of this product was used as a template for a nested reaction with primers DW1 and DW6. All these primers are specific to malarial parasites and do not amplify host DNA or that of other apicomplexan species.
The PCR product of 1 kb was sequenced by Big Dye Terminator v3.0 Cycle Sequencing Kit in ABI Genetic Analyzer (ABI, USA), using PCR oligonucleotides (DW1 and DW6) and internal primers DW3 and DW8 (Perkins and Schall, 2002). The sequence obtained was aligned using BLASTN (Basic Local Alignment Search Tool) with sequences from the GenBank available at http://www.ncbi.nlm.nih.gov/blast/Blast.cgi (Altschul et al., 1997) and MalAvi available at http://mbio-serv2.mbioekol.lu.se/Malavi/blast.html (Zhang et al., 2000). The P. nucleophilum sequence was deposited in the GenBank database with accession number JX467689.
2.4. Phylogenetic analysis
The phylogenetic relationship of the P. nucleophilum lineage from the Egyptian Goose and other Plasmodium species described in birds was inferred using cytb gene sequences. The sequences were selected from the MalAvi database, a public database of malaria parasites and related haemosporidians in avian hosts (Bensch et al., 2009). The phylogenetic tree was constructed using the Bayesian inference method implemented in MrBayes v3.2.0 (Huelsenbeck and Ronquist, 2001). Bayesian inference was performed with two Markov Chain Monte Carlo searches of 3 million generations each with sampling of 1 in 300 trees. After a burn-in of 25%, the remaining 15,002 trees were used to calculate the 50% majority-rule consensus tree.
3. Results and discussion
Co-infection of P. (Novyella) nucleophilum Manwell, 1935 (Fig. 1a–f) and Plasmodium (Haemamoeba) sp. (Fig. 1g–i) was detected by microscopy. The intensity of parasitemia of these parasites was 2.4% and 0.2%, respectively. These two species can be readily distinguished from each other due to the presence or absence of nucleophilic parasites and of large roundish trophozoites. P. nucleophilum is characterized by (i) small (less than erythrocyte nuclei) meronts with scanty cytoplasm; (ii) ⩽8 merozoites in meronts; (iii) small (length <10 μm on average) elongated gametocytes possessing <10 pigment granules; (iv) the presence of vacuoles and refractive globules in some trophozoites and meronts; (v) trophozoites, meronts and gametocytes appressed to erythrocyte nuclei (nucleophilic feature) (Fig. 1a–f). The parasites observed in the Egyptian Goose were similar to the blood stages present in the type material of P. nucleophilum. However, (i) small vacuoles were frequently observed in trophozoites and meronts in the blood of the goose, and (ii) refractive globules were observed in many erythrocytic meronts. These feature are also visible in the type material of P. nucleophilum, but were observed less frequently; this could be a peculiarity of the parasite development in the goose. In contrast, the second species identified, P. (Haemamoeba) sp., lacks nucleophilic blood stages and is characterized by large roundish trophozoites, each with a prominent centrally collated vacuole (Fig. 1g–i). Because large trophozoites (size close to erythrocyte nuclei), which displace the erythrocyte nuclei were present, this parasite belongs to the subgenus Haemamoeba (Fig. 1g). For definitive identification of P. (Haemamoeba) species, mature meronts and gametocytes are required; however, these blood stages were absent from the thin blood film.
Fig. 1.
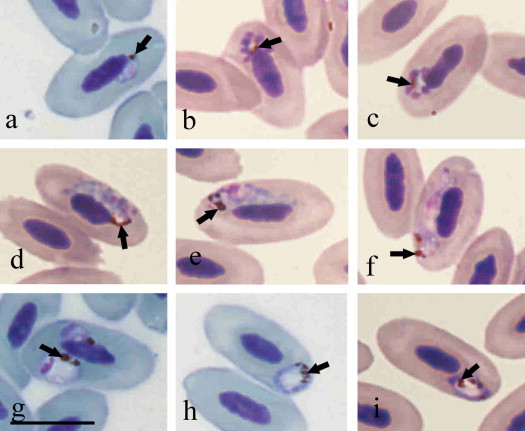
Photomicrographs of Plasmodium parasites visualized from thin blood smears obtained from an Egyptian Goose (Alopochen aegyptiacus) in São Paulo Zoo, Brazil. Characteristic of Plasmodium (Novyella) nucleophilum (lineage EG01, GenBank JX467689) the trophozoite (a), meronts (b–c), macrogametocytes (d, e), and microgametocyte (f) are appressed to erythrocyte nuclei (nucleophilic features). Plasmodium (Haemamoeba) sp. (g–i) lacks nucleophilic blood stages and possesses large roundish trophozoites, each with a prominent centrally located vacuole; pigment granules are gathered around the vacuoles. Note that early Plasmodium (H.) sp. trophozoites markedly displace erythrocyte nuclei (g). Arrows, pigment granules. Scale bar = 10 μm.
P. (Novyella) nucleophilum has been recorded in all zoogeographical regions, except the Australian and Antarctic, but is particularly frequently reported in the Americas. This parasite is particularly common in birds belonging to the Passeriformes, but has also been identified in species of Anseriformes, Columbiformes and Piciformes. Originally, P. nucleophilum was discovered in North America and has subsequently been reported in many bird species in South America (Manwell, 1935; Garnham, 1966). A sub-species, P. nucleophilum toucani, has been reported in Brazil in the Chestnut-mandibled Toucan (Piciformes), but its distribution has been insufficiently investigated (Manwell and Sessler, 1971). It is worth mentioning that Lucena (1939) identified P. nucleophilum in house sparrows (Passer domesticus) examined in Sao Paulo. It is probable that this bird serves as a natural reservoir host of P. nucleophilum-malaria infection, a fact that warrants further investigation. Blood stages of the reported parasite are morphologically similar to previous descriptions (Manwell, 1935; Valkiūnas, 2005), so their detailed morphological description was not provided. Virulence of P. nucleophilum varies markedly. Certain subspecies are relatively benign in experimentally infected canaries, but can be highly virulent in experimentally infected ducklings, and are fatal in many cases (Valkiūnas, 2005).
The Haemamoeba subgenus contains 11 malaria parasite species, and P. relictum, the type species, has been reported from São Paulo Zoo (Bueno et al., 2010). However, the P. (Haemamoeba) parasite recorded in this study is more similar to Plasmodium (Haemamoeba) tejerai because each of its advanced trophozoites and young erythrocytic meronts possessed a large vacuole with pigment granules arranged around it, the characteristic features of this species. Other described P. (Haemamoeba) parasites do not possess these characters (Valkiūnas, 2005). Plasmodium tejerai was first recorded in domestic turkey, Meleagris gallopavo, in Venezuela and some experimentally infected birds, including several Anatidae species (Gabaldon and Ulloa, 1977). Recently, it was identified in penguins from a rehabilitation centre in South Brazil (Silveira et al., 2013). It is the only Plasmodium parasite with large vacuoles and pigment granules arranged around the vacuoles in trophozoites (Fig. 1g–i) found in South America (Gabaldon and Ulloa, 1977). Because mature meronts and gametocytes of P. (Haemamoeba) sp. were absent from our blood sample and we were unable to detect sequence data, the definitive identification of the parasite is currently impossible.
The PCR protocol did not confirm co-infection and amplify only one cytb sequence, since no “double bases” were present in electropherograms. Previous studies have demonstrated that mixed infections are not efficiently detected using general primers (Valkiūnas et al., 2006; Martínez et al., 2009). Moreover, Plasmodium spp. detection depends on the parasite lineage composition during co-infections and the intensity of the infections. For haemosporidians, the PCR assays currently used can underestimate the occurrence of co-infections in the majority of natural infections (Valkiūnas et al, 2008a). In a recent study that used a PCR methodology to detect haemosporidians in 214 Brazilian birds, 14% of Plasmodium spp. infections were verified, but no co-infections were observed (Marzal et al., 2011). Failure to amplify the cytb gene of Plasmodium polymorphum in a mixed infection with P. relictum, even with a significantly greater parasitemia in the former, has also been demonstrated (Zehtindjiev et al., 2012). Thus, the combination of microscopy and PCR-based tools is essential for correct identification of Plasmodium spp. and investigation of the biodiversity of haemosporidian parasites in wildlife.
The sequence obtained (GenBank #JX467689, 1122 bp) was compared by BLASTN with GenBank and MalAvi sequences. In the GenBank database, the most similar Plasmodium sequences were GQ395660 and GQ141580 (both from Plasmodium sp.) with 97% identity (1006 of 1036 bp). The most similar sequence with species identification was a sequence from P. ashfordi (#AF254962) belonging to the subgenus Novyella, with 94% identity (451 of 478 bp). This sequence was formerly incorrectly attributed to P. nucleophilum (see review in Valkiūnas et al., 2007). In the GenBank, the presence of this and other misidentified sequences of avian haemosporidians has been proven (Valkiūnas et al., 2008b). The great majority of blood stages of P. ashfordi are non-nucleophilic, thus it can be readily distinguished from P. nucleophilum (Fig. 1).
In the MalAvi database, which comprises 1323 sequences and 590,601 total base pairs, we identified a sequence (AY640137), DENPET03 lineage of P. nucleophilum, with 100% identity in 479 bp (nucleotides 235–713 from our sequence). This sequence has been reported in 13 avian hosts belonging to 11 genera, 5 families and 2 orders (including passerines) in North and South America (Table 1). This is in agreement with previous microscopic studies (Garnham, 1966; Valkiūnas, 2005) and demonstrates that P. nucleophilum is likely a host generalist, as has been demonstrated for many other avian Plasmodium species (Szymanski and Lovette, 2005; Palinauskas et al., 2007). However, in the MalAvi database, there is no record of occurrence of any haemosporidian parasites in the Egyptian goose (http://mbio-serv2.mbioekol.lu.se/Malavi/index.html).
Table 1.
Avian hosts and countries where the DENPET03 lineage of Plasmodium nucleophilum has been reported.
| Order | Family | Species | Reference | Country |
|---|---|---|---|---|
| Basileuterus culicivorus | Durrant et al. (2006) | Uruguay | ||
| Basileuterus leucoblepharus | Durrant et al. (2006) | Uruguay | ||
| Cacicus cela | Durrant et al. (2006) | Guyana | ||
| Cacicus haemorrhous | Durrant et al. (2006) | Guyana | ||
| Cranioleuca pyrrhophia | Durrant et al. (2006) | Uruguay | ||
| Fringillidae | Geothlypis trichas | Pagenkopp et al. (2008) | USA | |
| Passeriformes | Gnorimopsar chopi | Durrant et al. (2006) | Uruguay | |
| Setophaga petechia | Szymanski and Lovette (2005) | USA | ||
| Volatinia jacarina | Durrant et al. (2006) | Guyana | ||
| Zonotrichia capensis | Durrant et al. (2006) | Uruguay | ||
| Passeridae | Passer domesticus | Marzal et al. (2011) | Brazila | |
| Vireonidae | Vireo griseus | Ricklefs and Fallon (2002) | USA | |
| Psittaciformes | Psittacidae | Diopsittaca nobilis | Durrant et al. (2006) | Guyana |
Midwest and North.
The phylogenetic analysis is in agreement with our morphological identification since the reported cytb sequence clustered with sequences of several Novyella species, forming a well-supported clade with high posterior probability (Fig. 2). This record was included in the MalAvi database as EG01 (acronym of Egyptian goose lineage 1).
Fig. 2.
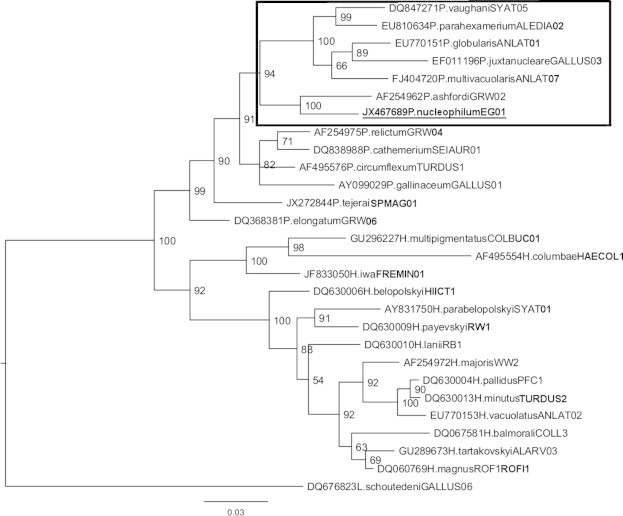
Bayesian phylogeny of cytochrome b gene lineages of species of avian haemosporidian parasites. A lineage recorded in the Egyptian Goose Alopochen aegyptiacus is provided underlined. Names of the lineages are given after the species names of parasites. GenBank accession numbers of the lineages are provided before the parasite species names. Nodal support values (in percentage) indicate posterior clade probabilities. Plasmodium species from Novyella subgenus are boxed.
Although in most organisms the gene of choice for barcoding is cytochrome oxidase subunit I (COI), a 479 bp fragment of the cytb gene has been successfully used as a barcode of avian haemosporidian species (Bensch et al., 2009). This study provides the first assignment of a mitochondrial cytb gene sequence to P. nucleophilum deposited in the GenBank and can be used for molecular identification (barcoding) of this infection in other birds. The same sequence has been reported in numerous bird species in the Americas (Table 1). The identification of barcodes to recognize avian haemosporidians species is an important step, since morphological identification of these parasites requires extensive taxonomic experience and is not always possible due to predominance of light parasitemias in wildlife (Hajibabaei et al., 2007). Currently, there are few experts with such knowledge and few people in the next generation of scientists are learning these taxonomic skills (Valkiūnas et al., 2008b); thus, the development of barcodes to identify avian malaria and related parasites is an urgent task.
Acknowledgments
The authors are grateful to the staff of the São Paulo Zoological Park Foundation (Fundação Parque Zoológico de São Paulo) for their support during this study. The authors would also like to thank Dr. A. Warren of the Natural History Museum, London, UK, for providing the neotype material of P. nucleophilum. This research was funded by FAPESP (2012/51427-1) to KK. All procedures were approved by the Ethical Principles in Animal Research, of the Ethics Committee of Institute of Tropical Medicine, University of São Paulo (CPE-IMT/193), and were in full compliance with federal permits issued by the Brazilian Ministry of the Environment (SISBIO # 34605-2).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch S., Stjernman M., Hasselquist D., Östman Ö., Hansson B., Westerdahl H., Pinheiro R.T. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. Biol. Sci. 2000;267:1583–1589. doi: 10.1098/rspb.2000.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch S., Hellgren O., Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Braga E.M., Silveira P., Belo N.O., Valkiūnas G. Recent advances in the study of avian malaria: an overview with an emphasis on the distribution of Plasmodium spp. in Brazil. Mem. Inst. Oswaldo Cruz. 2011;106(Suppl. I):3–11. doi: 10.1590/s0074-02762011000900002. [DOI] [PubMed] [Google Scholar]
- Bueno M.G., Lopez R.P.G., Menezes R.M.T., Costa-Nascimento M.J., Lima G.F.M.C., Araújo R.A.S., Guida F.J.V., Kirchgatter K. Identification of Plasmodium relictum causing mortality in penguins (Spheniscus magellanicus) from Sao Paulo Zoo. Brazil. Vet. Parasitol. 2010;173:123–127. doi: 10.1016/j.vetpar.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Durrant K.L., Beadell J.S., Ishtiaq F., Graves G.R., Olson S.L., Gering E., Peirce M.A., Milensky C.M., Schmidt B.K., Gebhard C., Fleischer R.C. Avian haematozoa in South America: a comparison of temperate and tropical zones. Ornithol. Monogr. 2006;60:98–111. [Google Scholar]
- Gabaldon A., Ulloa G. Plasmodium (Haemamoeba) tejerai sp. n. en pavo domestico (Melleagris gallopavo) del Venezuela. Bol. Dir. Malariol. Saneam. Ambient. 1977;17:255–273. [Google Scholar]
- Garnham P.C.C. Blackwell Scientific Publications; Oxford: 1966. Malaria Parasites and Other Haemosporidia; p. 1114. [Google Scholar]
- Godfrey R.D., Fedynich A.M., Pence D.B. Quantification of hematozoa in blood smears. J. Wildl. Dis. 1987;23:558–565. doi: 10.7589/0090-3558-23.4.558. [DOI] [PubMed] [Google Scholar]
- Guimarães A.E., Mello R.P., Lopes C.M., Gentile C. Ecology of mosquitoes (Diptera: culicidae) in areas of Serra do Mar State Park, State of São Paulo, Brazil. I – monthly frequency and climatic factors. Mem. Inst. Oswaldo Cruz. 2000;95:1–16. doi: 10.1590/s0074-02762000000100001. [DOI] [PubMed] [Google Scholar]
- Hajibabaei M., Singer G.A., Hebert P.D., Hickey D.A. DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 2007;23:167–172. doi: 10.1016/j.tig.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Lucena D.T. Officinas graphicos do Jornal do Commercio; Recife: 1939. Malaria aviaria: subsidios para sua sistematica e transmissão; p. 126. [Google Scholar]
- Manwell R.D. How many species of avian malaria parasites are there? Am. J. Trop. Med. 1935;15:265–283. [Google Scholar]
- Manwell R.D., Sessler G.J. Malaria parasites of toucans. J. Protozool. 1971;18:570–574. doi: 10.1111/j.1550-7408.1971.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Martínez J., Martínez-De La Puente J., Herrero J., Del Cerro S., Lobato E., Rivero-De Aguilar J., Vásquez R.A., Merino S. A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: on the inefficiency of general primers for detection of mixed infections. Parasitology. 2009;136:713–722. doi: 10.1017/S0031182009006118. [DOI] [PubMed] [Google Scholar]
- Marzal A., Ricklefs R.E., Valkiūnas G., Albayrak T., Arriero E., Bonneaud C., Czirják G.A., Ewen J., Hellgren O., Hořáková D., Iezhova T.A., Jensen H., Križanauskienė A., Lima M.R., de Lope F., Magnussen E., Martin L.B., Møller A.P., Palinauskas V., Pap P.L., Pérez-Tris J., Sehgal R.N., Soler M., Szöllosi E., Westerdahl H., Zetindjiev P., Bensch S. Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS One. 2011;6:e21905. doi: 10.1371/journal.pone.0021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenkopp K., Klicka J., Durrant K., Garvin J., Fleischer R. Geographic variation in malarial parasite lineages in the common yellow throat (Geothlypis trichas) Conserv. Genet. 2008;9:1577–1588. [Google Scholar]
- Palinauskas V., Kosarev V., Shapoval A., Bensch S., Valkiūnas G. Comparison of mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites of the subgenera Haemamoeba and Giovannolaia (Haemosporida: Plasmodiidae) Zootaxa. 2007;1626:39–50. [Google Scholar]
- Perkins S.L., Schall J.J. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Parasitol. 2002;88:972–978. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Primarck B.R., Rodrigues E. Planta; Londrina: 2001. Biologia da Conservação; p. 328. [Google Scholar]
- Quist, C.F., Cornish, T., Wyatt, R.D., 2007. Mycotoxicosis. In: Thomas, N.J., Hunter, D.B., Atkinson, C.T. (Eds.), Infectious Diseases of Wild Birds, Blackwell Publishing, Oxford, pp. 417–430.
- Ribeiro M.C., Metzger J.P., Martensen A.C., Ponzoni F.J., Hirota M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009;142:1141–1153. [Google Scholar]
- Ribeiro A.F., Urbinatti P.R., de Castro Duarte A.M., de Paula M.B., Pereira D.M., Mucci L.F., Fernandes A., de Mello M.H., de Matos Júnior M.O., de Oliveira R.C., Natal D., dos Santos Malafronte R. Mosquitoes in degraded and preserved areas of the Atlantic Forest and potential for vector-borne disease risk in the municipality of São Paulo. Brazil. J. Vector Ecol. 2012;37:316–324. doi: 10.1111/j.1948-7134.2012.00233.x. [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E., Fallon S.M. Diversification and host switching in avian malaria parasites. Proc. Biol. Sci. 2002;269:885–892. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, J.C.R., Corrêa, S.H.R., 2006. Manejo Sanitário e Biosseguridade. In: Cubas, Z.S., Silva, J.C.R., Catão-Dias, J.L. (Eds.), Tratado de Animais Selvagens – Medicina Veterinária, Roca, São Paulo, pp. 1226–1244.
- Silveira P., Belo N.O., Lacorte G.A., Kolesnikovas C.K., Vanstreels R.E., Steindel M., Catão-Dias J.L., Valkiūnas G., Braga E.M. Parasitological and new molecular-phylogenetic characterization of the malaria parasite Plasmodium tejerai in South American penguins. Parasitol. Int. 2013;62:165–171. doi: 10.1016/j.parint.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Szymanski M.M., Lovette I.J. High lineage diversity and host sharing of malarial parasites in a local avian assemblage. J. Parasitol. 2005;91:768–774. doi: 10.1645/GE-417R1.1. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G. CRC Press; New York: 2005. Avian Malaria Parasites and Other Haemosporidia; p. 935. [Google Scholar]
- Valkiūnas G., Bensch S., Iezhova T.A., Krizanauskiene A., Hellgren O., Bolshakov C. Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J. Parasitol. 2006;92:418–422. doi: 10.1645/GE-3547RN.1. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G., Zehtindjiev P., Hellgren O., Ilieva M., Iezhova T.A., Bensch S. Linkage between mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites, with a description of Plasmodium (Novyella) ashfordi sp. nov. Parasitol. Res. 2007;100:1311–1322. doi: 10.1007/s00436-006-0409-3. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G., Iezhova T.A., Krizanauskiene A., Palinauskas V., Sehgal R.N.M., Bensch S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J. Parasitol. 2008;94:1395–1401. doi: 10.1645/GE-1570.1. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G., Atkinson C.T., Bensch S., Sehgal R.N., Ricklefs R.E. Parasite misidentifications in genbank: how to minimize their number? Trends Parasitol. 2008;24:247–248. doi: 10.1016/j.pt.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Zehtindjiev P., Krizanauskiene A., Bensch S., Palinauskas V., Asghar M., Dimitrov D., Scebba S., Valkiūnas G. A new morphologically distinct avian malaria parasite that fails detection by established polymerase chain reaction-based protocols for amplification of the cytochrome b gene. J. Parasitol. 2012;98:657–665. doi: 10.1645/GE-3006.1. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Schwartz S., Wagner L., Miller W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]


