Keywords: Salmo trutta, Oncorhynchus mykiss, New Zealand, Parasite, Salmonid, Disease
Highlights
-
•
Native parasites are frequently acquired by exotic fish species.
-
•
Field surveys show that exotic salmonids are poor hosts of two native trematodes.
-
•
Salmonids have poor competency for native trematodes in experimental infections.
-
•
Exotic salmonids presence may decrease native parasite flow to native hosts.
Abstract
Exotic fish species frequently acquire native parasites despite the absence of closely related native hosts. They thus have the potential to affect native counterparts by altering native host–parasite dynamics. In New Zealand, exotic brown trout Salmo trutta and rainbow trout Oncorhynchus mykiss have acquired two native trematodes (Telogaster opisthorchis and Stegodexamene anguillae) from their native definitive host (the longfin eel Anguilla dieffenbachii). We used a combination of field surveys and experimental infections to determine the relative competence of native and exotic fish hosts for these native parasites. Field observations indicated that the longfin eel was the superior host for both parasites, although differences between native and exotic hosts were less apparent for S. anguillae. Experimental infections indicated that both parasites had poorer establishment and survival in salmonids, although some worms matured and attained similar sizes to those in eels before dying. Overall, the field surveys and experimental infections indicate that these exotic salmonids are poor hosts of both native trematodes and their presence may decrease native parasite flow to native hosts.
Introduction
Whether through accidental introductions or intentional releases, many species attain distributions beyond their natural ranges and form novel interactions with native counterparts. Interactions mediated by predation and competition are frequently cited as detrimental outcomes of the presence of exotic species in novel habitats (e.g. Ricciardi et al., 1998, Wiles et al., 2003). Increasingly, however, exotic–native interactions mediated by disease are also recognised (e.g. Van Riper et al., 2002, Tompkins et al., 2003, LaDeau et al., 2007). Exotic salmonid fishes, for example, have long been known to influence native communities through novel predatory and competitive interactions (Crowl et al., 1992, Townsend, 2003), while the possibility of additional interactions mediated by disease have only recently been highlighted (Tompkins and Poulin, 2006, Kelly et al., 2009a).
Although salmonids are native to cold water environments of the Northern Hemisphere, global translocation for angling and aquaculture (Crawford and Muir, 2008) has resulted in the exchange of fish between regions where native salmonids already occur (e.g. rainbow trout Oncorhynchus mykiss native to North America’s Pacific Coast introduced to the United Kingdom, and brown trout Salmo trutta native to Eurasia introduced to North America; MacCrimmon and Marshall, 1968, MacCrimmon, 1971) and the introduction of salmonids to regions where they were naturally absent (e.g. Australasia, South America). Salmonids introduced to the Southern Hemisphere have largely escaped salmonid-adapted diseases because the introduction of parasite-free ova or fry (Kennedy and Bush, 1994), and the use of multiple-stage translocations (e.g. New Zealand brown trout originated from fish introduced to Australia; MacCrimmon and Marshall, 1968), severely limited the likelihood of co-introducing diseases during translocations. However these fish have not escaped disease burdens entirely, with exotic salmonids acquiring generalist parasites from native fish communities (Dix, 1968, Ortubay et al., 1994) and, in some cases, attaining equal or greater parasite species richness than in their area of origin (Poulin and Mouillot, 2003).
The ease with which exotic salmonids acquire native parasites increases the potential for these fish to modify native host–parasite dynamics, either acting as competent parasite reservoirs that “spillback” infection to native hosts (see Daszak et al., 2000, Kelly et al., 2009b), or as incompetent parasite hosts that dilute disease burdens in native hosts (e.g. Telfer et al., 2005, Kopp and Jokela, 2007). While few studies have investigated the influence of exotic salmonids on native host–parasite dynamics, assessments of Acanthocephalus tumescens in rainbow trout (Paterson et al., 2013) and Acanthocephalus galaxii in brown trout (Paterson et al., 2011) suggest that exotic salmonid competence for native parasites often differs from that of the native hosts.
In Lake Pearson, New Zealand, the co-existence of brown and rainbow trout presents an opportunity to compare the competence of two exotic salmonids for native fish parasites simultaneously. Although knowledge of native host–parasite dynamics in this lake was lacking prior to the current study, the native fish community was known to be comprised of multiple hosts of the native trematodes Stegodexamene anguillae MacFarlane, 1951 and Telogaster opisthorchis MacFarlane, 1945. These trematodes co-infect their definitive native eel hosts, Anguilla spp. (S. anguillae – upper intestine, T. opisthorchis – lower intestine; MacFarlane, 1945, MacFarlane, 1951), and have been reported in exotic salmonids elsewhere in New Zealand (Dix, 1968, Hine et al., 2000, Kelly et al., 2009a). Both trematodes exhibit similar life cycles, with eggs released from adult worms reaching the water column with eel faeces, and the first intermediate host (the snail Potamopyrgus antipodarum) becoming infected by either ingesting eggs (for T. opisthorchis) or encountering free-living larvae that emerge from eggs (for S. anguillae). Second intermediate hosts, the native fish Galaxias spp. and Gobiomorphus spp., become infected by encountering cercarial infective stages released from snails that encyst as metacercariae in fish tissues. Eels acquire trematodes by consuming infected second intermediate hosts. Salmonids are known to prey on both Galaxias and Gobiomorphus in New Zealand lakes (e.g. McCarter, 1986, Rowe et al., 2002). Hence, they too frequently encounter trematode metacercarial cysts. Exotic salmonids may therefore modify native host–parasite dynamics by altering the abundance of second intermediate hosts through predation, in addition to altering disease burdens in both intermediate and definitive hosts.
Here we apply a three-pronged approach to (i) quantify the relative competence of native and exotic fish hosts for these two generalist native parasites and (ii) determine whether salmonid species differ in their influence on native host–parasite dynamics. First, we use field data to evaluate the relative acquisition of native trematodes by native and exotic hosts. Second, we use experimental infections to determine whether host competence for native parasites differs between exotic salmonids, and between native and exotic hosts. Third, we consider how the acquisition of native parasites by exotic salmonids may influence native host–parasite dynamics.
Materials and methods
Study site
Lake Pearson (24°10′520E, 57°87′570N) is a small, shallow lake (1.79 km2, maximum depth 17 m) situated in the Waimakariri River Catchment, South Island, New Zealand. The fish community is comprised of four native fish species (longfin eel Anguilla dieffenbachii, common bully Gobiomorphus cotidianus, upland bully Gobiomorphus breviceps and koaro Galaxias brevipinnis), and four exotic salmonids (brown trout, rainbow trout, chinook salmon Oncorhynchus tshawytscha and lake trout Salvelinus namaycush), though the latter two salmonids are considered rare (Hutchison, 1981, Rowe and Graynoth, 2002).
Field surveys
Field surveys were conducted during April–May (Austral Autumn) and September 2008 (Austral Spring) to determine the prevalence (proportion of infected individuals) and infection intensity (number of parasites per infected host; Bush et al., 1997) of T. opisthorchis and S. anguillae in second intermediate and definitive fish hosts. Salmonids were sampled using sinking 25 m multi-panel gill nets (mesh sizes: 13, 25, 38, 56, 70 mm) set for 1–2 h during daylight hours, while longfin eels were sampled using unbaited fyke nets (wing length 450 cm, stretched mesh size 20 mm) set overnight. Koaro and bullies were sampled using unbaited minnow traps (height 25 cm, length 45 cm, stretched mesh size 5 mm) set overnight, with seine nets used to capture additional fish when minnow traps obtained fewer than 30 individuals/species/survey. The number of fish captured by each sampling technique was used to determine the relative abundance ratios of fish hosts, comparable only between species caught using the same sampling technique.
A random sub-sample of up to 30 fish per species per sampling period was euthanized (totals: 47 brown trout, 42 rainbow trout, 40 common bully, three upland bully, two koaro). Fork length (FL mm; salmonids) or total length (TL mm; native fish) were recorded for each fish prior to preserving the alimentary canal (oesophagus to anus) in 10% buffered formalin. Remaining body tissues of bullies and koaro were frozen for later examination for metacercarial cysts. Body tissues of salmonids were not examined for metacercarial cysts because previous field surveys showed brown trout had very low prevalence and infection intensity of metacercarial cysts, although sympatric native fish had moderate infections (Kelly et al., 2009a). Longfin eels were not examined for intestinal parasites and were instead released alive, as this native fish is of high conservation status in Lake Pearson. To provide a reference for trematode prevalence, infection intensity and maturity in native definitive hosts, nine longfin eels were collected from a nearby lake that supported the same fish species as Lake Pearson (Lake Sumner; 24°42′440E, 58°33′810N).
Trematodes in the alimentary canals of trout and eels were enumerated in the laboratory. The development status (non-gravid or gravid, based on the presence of eggs in utero) and worm size (calculated from the surface area of an ellipse = π × (L/2) × (W/2), L = length in μm and W = width at widest point in μm) were determined for each worm. The number of eggs in the uterus and egg volume (V = π × L × W2/6, where L = length and W = width) of a subsample of five eggs were measured for each gravid worm. Stomach contents of each definitive host were examined to estimate the number of koaro and bullies consumed.
The density of the snail P. antipodarum was estimated from 12 benthos samples (706 cm2) collected from random locations within 5 m of the shore during April–May 2008 (n = 12), with samples preserved in 10% formalin prior to enumeration of snails.
Experimental infection
An infection experiment was conducted to evaluate rates of parasite establishment, fecundity and mortality for both trematode species in native and exotic definitive hosts. Brown trout (FL 107–148 mm) were collected by electric fishing from the Cap Burn (22°95′560E, 55°46′220N), Otago. Rainbow trout (FL 182–244 mm) were obtained from Otago Fish and Game’s Macraes Hatchery, Otago. Wild-caught longfin eels (TL 460–550 mm) were sourced from New Zealand Eels Limited, Te Kauwhata, Waikato. All fish were acclimatised in separate 200 L tanks in the University of Otago’s controlled climate facilities (13 h day/11 h night period, 10 °C, 15% daily water change) for 2 weeks prior to experimentation. A common food consumed by all fish species was not available so fish were maintained on different diets fed ad libitum (longfin eel and brown trout – blood worms; rainbow trout – commercial fish pellets).
Experimental fish were treated during acclimatisation with an anthelmintic, Praziquantel, in tablet form (25 mg – longfin eel; 12.5 mg – rainbow and brown trout). The anthelmintic was administered orally to fish anaesthetised in a benzocaine solution (100 ppm), with fish placed in a tank of aerated freshwater for 5 min post-recovery to ensure regurgitation did not occur. Experimental fish were maintained for a further 7 days prior to experimental infection, to allow for the elimination of residual anthelmintic from body tissues and its excretion into tank water (Björklund and Bylund, 1987). The reabsorption of active ingredients excreted from fish was minimised through daily water changes. Anthelmintic efficacy was tested by assessing parasite infections in a sub-set of five treated and untreated fish per species. Treated and untreated fish were autopsied 48 h post-anthelmintic exposure, with no intestinal parasites recovered in brown or rainbow trout in either treatment. Intestinal parasites were also absent from anthelmintic-treated eels, while all untreated eels contained trematodes (mean infection intensity of both species combined = 64 adult trematodes per individual).
For each trematode species, five experimental fish of each species were randomly assigned to three infection time periods (5, 15 or 25 days, based on relationship between adult size and age at first reproduction; Trouve et al., 1998) and placed into individual 30 L tanks without food for 48 h prior to experimentation. Common bully (n = 120), naturally infected with T. opisthorchis (mean intensity 43.2) and S. anguillae metacercariae (mean intensity 25.0), were collected from Lake Waihola (22°84′950E, 54°61′500N), Otago, and transported live to the laboratory. Live metacercariae were carefully removed from the body tissues of freshly euthanized fish and placed into gelatine capsules (size 0–00) at densities reflecting the average infection intensity of trematodes in bullies of Lake Pearson (40–50 S. anguillae, or 80–100 T. opisthorchis). As progenetic reproduction can occur in both trematode species (MacFarlane, 1945, MacFarlane, 1951), whereby eggs are produced while trematodes are encysted in second intermediate hosts, metacercarial cysts with progenetic eggs were omitted from the experiment. Capsules were administered to anaesthetised fish as for the anthelmintic treatment, with all infected fish maintained in individual tanks on their respective diets post-infection. At the completion of each infection period (5, 15 or 25 days), fish were euthanized and immediately examined for parasites by removing the alimentary canal from oesophagus to anus and splitting it longitudinally. The number of trematodes was noted prior to fixing the parasites in 10% buffered formalin. Worm measurements were made as in the field survey.
Statistical analyses
Statistical analyses were conducted using R (version 2.13.1, R Core Team, 2011). For field and experimental infection data, non-parametric analyses (Mann–Whitney–Wilcoxon and Kruskal–Wallis) were used because assumptions for parametric tests could not be met (e.g. unequal sample sizes and/or variances), with samples also pooled across seasons to increase sample size. Post-hoc tests were used to assess where differences existed when variance was significant between more than two species. In experimental infections, establishment was only assessed at 5 days post-infection between species.
Parasite flow
Parasite flow diagrams were constructed to graphically assess the potential influence of exotic salmonid presence on T. opisthorchis and S. anguillae in their native hosts. Although a parasite’s fitness can be negatively influenced by the presence of another parasite species (e.g. Holland, 1984, Poulin et al., 2003), and both trematodes co-infect the same second intermediate and definitive host species, each trematode was evaluated separately because T. opisthorchis and S. anguillae occupy different parts of the eel’s gut (Macfarlane, 1952) and their occurrence in fish is positively correlated (Hine and Francis, 1980). It thus seems safe to assume that T. opisthorchis and S. anguillae do not compete for space in the gut, which could otherwise have resulted in their lowered establishment, growth and maturity in each other’s presence.
Common and upland bully could not be distinguished in the salmonid gut content analysis. We therefore assumed that the rate at which definitive hosts acquire infection from each bully species is proportional to the relative abundance of the bully species in the lake. Mortality of T. opisthorchis in longfin eels could not be parameterised from infection experiments, as worms survived beyond 25 days post-infection and did not show decreasing infection intensity with time. Rather, mortality was calculated from a regression of longevity of adult trematodes against their body size (y = 260.63x − 777.99), based on a compilation of platyhelminth life history data (Trouve et al., 1998).
Results
Field surveys
Two exotic salmonid species, brown trout (n = 71 [47 dissected for parasites], FL 117–610 mm) and rainbow trout (n = 42 [42], FL 87–536 mm) were captured in Lake Pearson (ratio 2.6:1, gill nets). Three native fish species were captured by minnow trap; common bully (n = 290 [40], TL 20–114 mm), upland bully (n = 40 [3], TL 26–59 mm) and koaro (n = 2 [2], TL 39–65 mm; ratio 95:13:1). Fyke nets captured only longfin eel in both lakes, with eels in Lake Sumner (n = 19 [14], TL 403–1055 mm) tending to be slightly larger than those in Lake Pearson (n = 16, TL 405–830 mm; Mann–Whitney–Wilcoxon test W = 69, P = 0.006).
Telogaster opisthorchis
Overall, T. opisthorchis prevalence was highest in longfin eel (81.8–66.7%) compared to brown (17–67%) and rainbow trout (22–33%; Table 1). Infection intensity was greater in longfin eel than rainbow trout (Kruskal–Wallis X2 = 12.16, P = 0.002). Worm size differed between species, with the largest worms in eels and the smallest worms in brown trout (Kruskal–Wallis X2 = 119.33, P < 0.0001; Fig. 1a). The majority of worms in longfin eels were gravid (80–83%; Table 1) and contained relatively high numbers of eggs (228.7–564.6 eggs). Few worms were gravid in brown (2%) or rainbow trout (0–4%) and contained relatively few eggs (10–13 and 3.5 eggs, respectively; Fig. 1c). Additionally, T. opisthorchis eggs tended to be larger in longfin eel than in either salmonid species (Kruskal–Wallis X2 = 36.60, P < 0.0001; Fig. 1e).
Table 1.
Seasonal prevalence, intensity and reproductive status of the trematodes Telogaster opisthorchis and Stegodexamene anguillae naturally infecting exotic salmonids (Lake Pearson) and native longfin eel (Lake Sumner).
| Parasite species | Host | No. of hosts | Season | Prevalence % (CI) | Mean intensity (±SE) | Gravid (%) |
|---|---|---|---|---|---|---|
| T. opisthorchis | BT | 32 | Pre-winter | 22 (9.9–40.4) | 10.57 ± 6.19 | 2 |
| 15 | Post-winter | 33 (13.0–61.3) | 10.20 ± 5.09 | 2 | ||
| RT | 30 | Pre-winter | 17 (6.3–35.5) | 4.00 ± 1.48 | 0 | |
| 12 | Post-winter | 67 (35.4–88.7) | 5.14 ± 2.09 | 4 | ||
| LE | 11 | Pre-winter | 82 (47.8–96.8)⁎ | 62.5 ± 27.9 | 80 | |
| 3 | Post-winter | 67 (12.5–98.2)⁎ | 25 ± 17 | 83 | ||
| S. anguillae | BT | 32 | Pre-winter | 9 (2.5–26.2) | 1.67 ± 0.33 | 10 |
| 15 | Post-winter | 53 (27.4–77.8) | 3.43 ± 1.00 | 14 | ||
| RT | 30 | Pre-winter | 17 (6.3–35.5) | 7.4 ± 4.11 | 3 | |
| 12 | Post-winter | 67 (35.4–88.7) | 2.71 ± 0.57 | 13 | ||
| LE | 11 | Pre-winter | 63.6 (31.6–87.6) | 18.4 ± 5.8 | 85 | |
| 3 | Post-winter | 100 (31.0 – 100) | 7 ± 3.5 | 83 | ||
Note: BT – brown trout, RT – rainbow trout, LE – longfin eel (Lake Sumner).
Significant differences (pooled for season, P < 0.01).
Fig. 1.
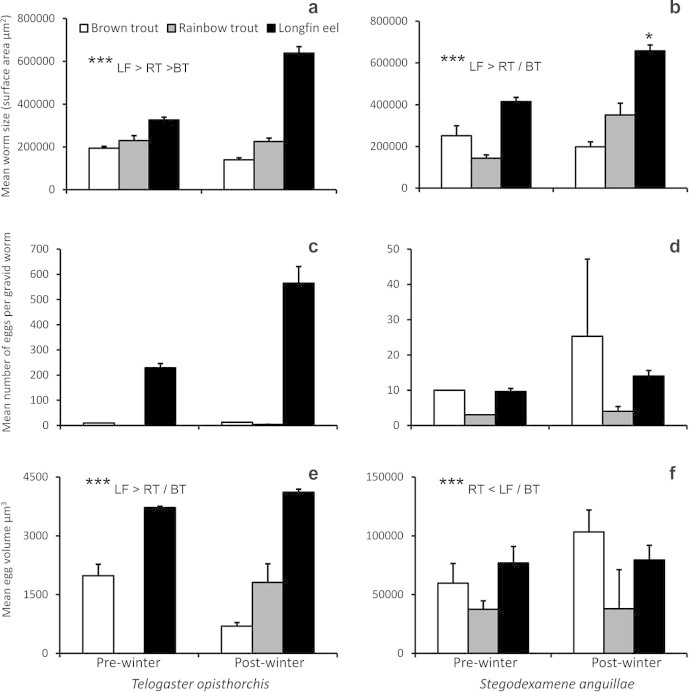
Mean worm size, number of eggs and egg volume of the trematodes Telogaster opisthorchis (a, c, e) and Stegodexamene anguillae (b, d, f) naturally infecting exotic salmonids (Lake Pearson) and native longfin eel (Lake Sumner). Error bars indicate standard error, ∗∗∗Significant differences (P < 0.0001).
Stegodexamene anguillae
Prevalence of S. anguillae tended to increase in all hosts over winter, with highest prevalence recorded in the relatively few longfin eels captured (n = 9–3, 63.6–100%, respectively; Table 1). Infection intensity did not differ between hosts (Kruskal–Wallis X2 = 3.41, P = 0.182). Worms recovered from longfin eels were larger than those in either salmonid (Kruskal–Wallis X2 = 76.94, P < 0.002; Fig. 1b). The majority of S. anguillae in longfin eels were gravid (85–83%, 9.6–14 eggs/worm), while few gravid worms were present in salmonids (rainbow trout 3–13%, 3–4 eggs/worm; brown trout 10–14%, 10–25.3 eggs/worm; Fig. 1d). Egg volume was significantly smaller in rainbow trout than the other fish species (Kruskal–Wallis species X2 = 31.40, P < 0.0001; Fig. 1f).
Definitive host diet
Bullies (common and upland) were the main fish type consumed by salmonids, with the percentage of stomachs containing bullies increasing over winter (rainbow trout 43–67%; brown trout 26–53%). Brown trout contained an average of 2.4–2.9 bullies/stomach, while rainbow trout contained 1.7–4.3 bullies. Koaro were present in fewer than 3.2–6.7% of brown trout (mean intensity 2–3 koaro/stomach) and 20–7% of rainbow trout (mean intensity 2.3–1 koaro/stomach) between seasons. Longfin eel stomachs from Lake Sumner were empty or contained P. antipodarum snails.
Intermediate hosts
Of the three potential second intermediate hosts of T. opisthorchis and S. anguillae captured in Lake Pearson, common bully was most abundant (87%, n = 290), with the remaining host community comprised of upland bully (12%, n = 40) and koaro (1%, n = 2). Common bully were infected with both trematodes (prevalence: T. opisthorchis 32. 5%, S. anguillae 25%), with T. opisthorchis also present in upland bully and S. anguillae in koaro (Table 2).
Table 2.
Prevalence and infection intensity of Telogaster opisthorchis and Stegodexamene anguillae metacercariae in second intermediate hosts from Lake Pearson.
| Parasite species | Host | No. of hosts | Prevalence % (CI) | Mean intensity (±SE) |
|---|---|---|---|---|
| T. opisthorchis | Common bully | 40 | 32.5 (19.1–49.2) | 1.31 ± 0.17 |
| Upland bully | 3 | 66.7 (12.5–98.2) | 3 ± 0 | |
| Koaro | 2 | 0 (0–80.2) | – | |
| S. anguillae | Common bully | 40 | 25 (13.2–41.5) | 2.5 ± 0.5 |
| Upland bully | 3 | 0 (0–69.0) | – | |
| Koaro | 2 | 50 (9.4–90.5) | 2 | |
Snails were found in all benthic samples, with an average of 631.8 snails/m2, of which an average of 50.1% were larger than the minimum of 3.6 mm shell length recorded for infected snails (Macfarlane, 1952).
Experimental infection
Telogaster opisthorchis
Establishment of T. opisthorchis did not differ among host species at 5 days post-infection (Kruskal–Wallis X2 = 1.60, P = 0.499), with many individuals failing to acquire infection (Table 3). Worms survived in longfin eels until 25 days post-infection, while no worms were present in salmonids at 15 days post-infection. Worm size did not differ between brown trout and longfin eels at 5 days post-infection (Kruskal–Wallis X1 = 0.48, P = 0.490; Fig. 2a). At 5 days post-infection, one of the two worms present in rainbow trout contained mature eggs, while no gravid worms were present in brown trout or longfin eel. The percentage of gravid worms in longfin eel increased with infection time, with 66% of worms gravid at 25 days post-infection. Egg volume of worms from longfin eel also increased with time since infection (Kruskal–Wallis 5 vs. 15 days X1 = 4.61, P = 0.03; Table 3).
Table 3.
Prevalence, infection intensity, size and reproductive status of the trematodes Telogaster opisthorchis and Stegodexamene anguillae in experimentally infected exotic brown trout and rainbow trout, and native longfin eel.
| Parasite species | Host | No. of hosts | Day | Prevalence (%) | Establishment (%) (mean ± SE) | Mean intensity (±SE) | Gravid (%) | Egg numbers (mean ± SE) | Egg size μm3 (mean ± SE) |
|---|---|---|---|---|---|---|---|---|---|
| T. opisthorchis | BT | 5 | 5 | 60 | 3.7 ± 2.4 | 4.33 ± 2.4 | 0 | – | – |
| 5 | 15 | 0 | – | – | – | – | – | ||
| RT | 5 | 5 | 20 | 0.42 ± 0.4 | 2 | 50 | 8 | 3251 ± 1660 | |
| 5 | 15 | 0 | – | – | – | – | – | ||
| LF | 4 | 5 | 40 | 8.04 ± 6.5 | 17.5 ± 11.5 | 0 | – | – | |
| 5 | 15 | 40 | 19.72 ± 13.2 | 47 ± 16 | 26 | 28.0 ± 4.2 | 3628 ± 119 | ||
| 4 | 25 | 40 | 26.75 ± 18.9 | 53.5 ± 13.5 | 66 | 42.9 ± 4.7 | 3915 ± 84 | ||
| S. anguillae | BT | 5 | 5 | 20 | 0.67 | 1 | 0 | – | – |
| 5 | 15 | 20 | 1.33 | 2 | 50 | 11 | 84581 ± 7044 | ||
| RT | 5 | 5 | 20 | 0.47 | 1 | 0 | – | – | |
| 4 | 15 | 0 | – | – | – | – | – | ||
| LF | 4 | 5 | 25 | 6.67 | 8 | 25 | 7.5 ± 4.5 | 25895 ± 5096 | |
| 5 | 15 | 40 | 18.33 ± 24.4 | 11 ± 10 | 60 | 10.9 ± 1.5 | 59348 ± 1694 | ||
| 3 | 25 | 0 | – | – | – | – | – | ||
Note: BT – brown trout, RT – rainbow trout, LE – longfin eel.
Fig. 2.
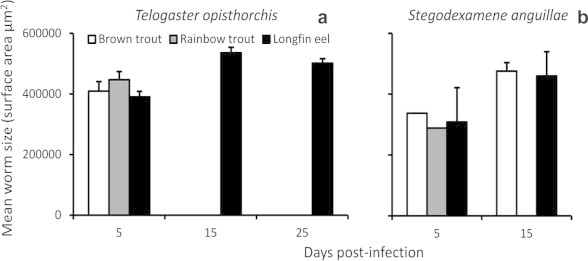
Mean worm size of the trematodes Telogaster opisthorchis (a) and Stegodexamene anguillae (b) in experimentally infected exotic brown trout and rainbow trout, and native longfin eel. Error bars indicate standard error.
Stegodexamene anguillae
Few fish became infected with S. anguillae, as evidenced by poor prevalence and establishment success in all species (Table 3). Highest establishment success occurred in longfin eel, with 6.7% of worms establishing at 5 days and 18.3% at 15 days post-infection. Worms were absent from rainbow trout at 15 days post-infection. Worm size showed little variation between host species at 5 and 15 days post-infection (Fig. 2b). Twenty-five percent of worms in longfin eel (n = 8) were gravid 5 days post-infection, increasing to 60% (n = 15) at 15 days post-infection. A single gravid worm was also present in brown trout 15 days post-infection, with no gravid worms observed in rainbow trout. Eggs present in brown trout were larger than those in longfin eel at 15 days post-infection (Kruskal–Wallis X1 = 9.32, P = 0.002, Table 3).
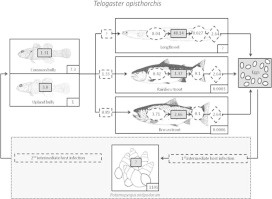
Parasite flow
Telogaster opisthorchis
Higher rates of parasite establishment and maturation and lower parasite mortality in longfin eel show that eels are the most successful hosts of T. opisthorchis, as indicated by higher parasite abundance in the field (Fig. 3a). Of the two salmonid species in Lake Pearson, brown trout is likely to have greater influence on T. opisthorchis dynamics, because this host has greater host abundance and parasite establishment as shown by the higher infection levels observed in the field. The majority of infections from second intermediate to definitive hosts originate from common bully, as this host is seven times more abundant than upland bully. Mean predation rates on bullies indicate that rainbow trout encounter more trematodes than brown trout, but the lower establishment rate of T. opisthorchis in rainbow trout results in fewer parasites in this salmonid host. However, as the relative density of rainbow trout in relation to longfin eel is unknown, it is difficult to determine how the contrasting parasite competence of native and exotic hosts will manifest in altered parasite dynamics.
Fig. 3.
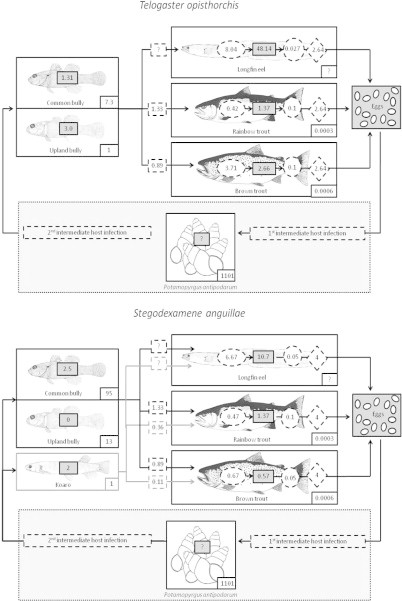
Flowchart summarising the circulation and transmission dynamics of trematodes, (a) Telogaster opisthorchis and (b) Stegodexamene anguillae, in native and exotic hosts in Lake Pearson. (Native fish images; McDowall, 2000; exotic fish; Rauque et al., 2003.)
Stegodexamene anguillae
Higher S. anguillae establishment rates in longfin eels than in the salmonids are also reflected in higher parasite abundance in the native host (Fig. 3b). Field surveys suggest that this parasite can mature in both salmonid species (as verified experimentally in brown trout), although poor survival of worms in salmonids means they are unlikely to contribute significantly to the parasite population. Worms establish at a lower rate in rainbow trout than brown trout, but rainbow trout consume more second intermediate hosts than brown trout. Thus, rainbow trout encounter more metacercarial cysts, resulting in higher parasite abundance in this host. Common bullies are the major source of S. anguillae infection for salmonids, due to this host’s high relative abundance and high predation rates by the definitive hosts.
Discussion
We used a combination of field surveys and experimental infections to determine the relative competence of native and exotic fish hosts for two native trematodes. We also used field and experimental results to assess likely parasite flows through the system. In general, field surveys demonstrated that longfin eel was the superior host of both trematodes, though some similarities were observed in S. anguillae infections between eels and salmonids. Experimental infections showed that both trematode species had poorer establishment and survival in salmonids, though some worms matured and attained similar sizes to those in eels prior to dying. Overall, both field surveys and experimental infections indicated that salmonids are unlikely to increase native parasite flow to native hosts by amplifying infection rates.
Of the two salmonid species in Lake Pearson, brown trout is likely to make the greater contribution to the populations of both parasites, due to its higher relative host density, infection intensity and establishment rate. Rainbow trout are more likely to act as a parasite sink because they consume a greater proportion of the second intermediate host fish, and worms have poorer establishment and survival in this host compared to both brown trout and longfin eel. Poorer establishment and survival of trematodes in salmonids compared with longfin eels appear to be the main reasons why salmonids are inferior hosts, as trematodes which do persist in salmonids achieve similar sizes to those in eels.
Although our study showed that salmonids had poor competence for native trematodes, the potential for salmonids to dilute infection in native hosts cannot be inferred solely from this. Relative densities of exotic and native hosts strongly influence whether native parasite dynamics will be altered by the presence of an exotic host (Paterson et al., 2011). Whilst the densities of longfin eel in Lake Pearson are unknown, previous studies of shared native parasites from native-exotic host complexes allow us to consider how exotic salmonids may influence parasite dynamics under given relative host densities. For example, if the density of salmonids relative to longfin eels is low, infection in native hosts may remain unchanged (as the density of salmonids would be too small for the poor parasite competence of salmonids to influence the overall parasite transmission dynamics). Alternatively, equal or greater density of salmonids in relation to longfin eels may reduce infection in native hosts, as the poor competence of exotic salmonids for native parasites could decrease the overall egg output from definitive hosts.
Infection dilution in native hosts, driven by the poor host competence of an exotic host for a shared native parasite, may only occur if infection to native hosts becomes limiting. Although parasites that encounter salmonids, following the consumption of their second intermediate host, are effectively removed from the parasite populations due to the poor competence of salmonids, it is common for a large proportion of infected second intermediate hosts not to encounter their definitive hosts, due, for example, to the effects of natural mortality or parasite induced mortality (Poulin, 2007). Hence, the consumption of second intermediate hosts by salmonids that were unlikely to encounter longfin eels may not alter parasite transmission to the native host. However, if infection from the second intermediate host population is limiting, either through naturally low host abundance prior to salmonid introduction, or driven by resource competition from salmonids, eels may experience reduced infection. Infection rates in intermediate hosts may also remain unchanged if the lowered infection intensity of trematodes supported by eels can still provide sufficient parasite eggs to maintain infection in the first intermediate host. Additionally, trematodes reproduce asexually in their first intermediate host, producing thousands of cercariae to increase their transmission to second intermediate hosts; the effect of a reduction in egg output from definitive hosts may be trivial when compared with the chance of cercariae encountering second intermediate hosts.
We are mindful that the conclusions drawn from this study must be treated with some caution due to small field survey sample sizes in some instances, lack of lake replication, and our need to obtain definitive eel hosts from an alternative lake. Although fewer eels were captured from Lake Sumner than salmonids from Lake Pearson, this native definitive host provides us with an indication of the prevalence and infection intensity that could be expected in the main study lake. Similarly prevalence and infection intensity estimates from upland bully and koaro are weakened by low sample sizes; however, given that these species are less abundant than common bully, they are less likely to strongly influence parasite dynamics. Additionally, the lack of replication at the lake scale is overcome by the use of experimental infections that provide some generality of what is likely to be occurring in lake systems, independent of lake-specific effects. We emphasise that understanding how exotic species influence native parasite dynamics may not be as simple as sampling hosts in the field or experimentally determining host competence for parasites, as field surveys and experimental infections do not take into account the influence of host densities on parasite dynamics. Furthermore, it may be unwise to generalise trends from previous studies of the influence of exotic salmonids on native parasites because they may falsely indicate that an exotic species will be a competent host for a native parasite. This study shows that the risks posed by exotic species that have acquired native parasites need to be evaluated on a case-by-case basis.
Acknowledgements
We thank T. Bayer, D. Kelly, A. Koone, C. Lagrue and C. Winkworth for assistance in the field. The Royal Society of New Zealand Marsden fund, and the Ministry of Business, Innovation and Employment Capability Funding supported this research. The manuscript was greatly improved by the comments of three anonymous reviewers.
References
- Björklund H., Bylund G. Absorption, distribution and excretion of the anthelmintic praziquantel (Droncit) in rainbow trout (Salmo gairdneri R.) Parasitol. Res. 1987;73 doi: 10.1007/BF00578511. [DOI] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Crawford S.S., Muir A.M. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Rev. Fish Biol. Fisher. 2008;18:313–344. [Google Scholar]
- Crowl T.A., Townsend C.R., McIntosh A.R. The impact of introduced brown and rainbow trout on native fish: the case of Australasia. Rev. Fish Biol. Fisher. 1992;2:217–241. [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.N. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Dix T.G. Helminth parasites of brown trout (Salmo trutta L.) in Canterbury, New Zealand. New Zeal. J. Mar. Fresh. 1968;2:363–374. [Google Scholar]
- Hine P.M., Francis R.I.C.C. Distribution of helminths in the digestive tracts of New Zealand freshwater eels: 3. Interspecific associations and conclusions. New Zeal. J. Mar. Fresh. 1980;14:349–356. [Google Scholar]
- Hine P.M., Jones J.B., Diggles B.K. National Institute of Water and Atmospheric Research; 2000. A Checklist of the Parasites of New Zealand Fishes, Including Previously Unpublished Records. [Google Scholar]
- Holland C. Interactions between Moniliformis (Acanthocephala) and Nippostrongylus (Nematoda) in the small intestine of laboratory rats. Parasitology. 1984;88:303–315. [PubMed] [Google Scholar]
- Hutchison D. A future for lake trout. Freshwat. Catch. 1981;11:4–5. [Google Scholar]
- Kelly D.W., Paterson R.A., Townsend C.R., Poulin R., Tompkins D.M. Has the introduction of brown trout altered disease patterns in native New Zealand fish? Freshwat. Biol. 2009;54:1805–1818. [Google Scholar]
- Kelly D.W., Paterson R.A., Townsend C.R., Poulin R., Tompkins D.M. Parasite spillback: a neglected concept in invasion ecology? Ecology. 2009;90:2047–2056. doi: 10.1890/08-1085.1. [DOI] [PubMed] [Google Scholar]
- Kennedy C.R., Bush A.O. The relationship between pattern and scale in parasite communities: a stranger in a strange land. Parasitology. 1994;109:187–196. doi: 10.1017/s0031182000076290. [DOI] [PubMed] [Google Scholar]
- Kopp K., Jokela J. Resistant invaders can convey benefits to native species. Oikos. 2007;116:295–301. [Google Scholar]
- LaDeau S.L., Kilpatrick A.M., Marra P.P. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710–713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- MacCrimmon H., Marshall T.L. World distribution of brown trout Salmo trutta. Can. J. Fish. Aquat. Sci. 1968;25:2527–2548. [Google Scholar]
- MacCrimmon H.R. World distribution of rainbow trout (Salmo gairdneri) Can. J. Fish. Aquat. Sci. 1971;28:663–704. [Google Scholar]
- Macfarlane M.V. Bionomics of two trematode parasites of New Zealand eels. J. Parasitol. 1952;38:391–397. [PubMed] [Google Scholar]
- MacFarlane W.V. The lifecycle of the heterophyoid trematode Telogaster opisthorchis n.g., n.sp. T. R. Soc. New Zeal. 1945;75:218–230. [Google Scholar]
- MacFarlane W.V. The life cycle of Stegodexamene anguillae n.g., n.sp., an allocreadiid trematode from New Zealand. Parasitology. 1951;41:1–10. doi: 10.1017/s0031182000016516. [DOI] [PubMed] [Google Scholar]
- McCarter N.H. Food and energy in the diet of brown and rainbow trout from Lake Benmore, New Zealand. New Zeal. J. Mar. Freshwat. Res. 1986;20:551–559. [Google Scholar]
- McDowall R.M. Raupo Publishing; New Zealand: 2000. The Reed Field Guide to New Zealand Freshwater Fishes. [Google Scholar]
- Ortubay S.G., Semenas L.G., Ubeda C.A., Quaggiotto A.E., Viozzi G.P. Direccion de Pesca; Subsecreteria de Recursos Naturales, Provincia de Rio Negro, Argentina: 1994. Catalogo de peces dulceacuicolas de la Patagonia Argentina y sus parasitos metazoos. [Google Scholar]
- Paterson R.A., Townsend C.R., Poulin R., Tompkins D.T. Introduced brown trout alter native acanthocephalan infections in native fish. J. Anim. Ecol. 2011;80:990–998. doi: 10.1111/j.1365-2656.2011.01834.x. [DOI] [PubMed] [Google Scholar]
- Paterson, R.A., Rauque, C.A., Fernandez, M.V., Townsend, C.R., Poulin, R., Tompkins, D.M., 2013. Native fish avoid parasite spillback from multiple exotic hosts: consequences of host density and parasite competency. Biol. Invas. 10.1007/s10530-013-0445-8. [DOI]
- Poulin R., Giari L., Simoni E., Dezfuli B.S. Effects of conspecifics and heterospecifics on individual worm mass in four helminth species parasitic in fish. Parasitol. Res. 2003;90:143–147. doi: 10.1007/s00436-002-0778-1. [DOI] [PubMed] [Google Scholar]
- Poulin R. second ed. Princeton University Press; Princeton: 2007. Evolutionary Ecology of Parasites. [Google Scholar]
- Poulin R., Mouillot D. Host introductions and the geography of parasite taxonomic diversity. J. Biogeogr. 2003;30:837–845. [Google Scholar]
- R Core Team, 2011. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
- Rauque C.A., Viozzi G.P., Semenas L.G. Component population study of Acanthocephalus tumescens (Acanthocephala) in fishes from Lake Moreno, Argentina. Folia Parasitol. 2003;50:72–78. doi: 10.14411/fp.2003.013. [DOI] [PubMed] [Google Scholar]
- Ricciardi A., Neves R.J., Rasmussen J.B. Impending extinctions of North American freshwater mussels (Unionoida) following the zebra mussel (Dreissena polymorpha) invasion. J. Anim. Ecol. 1998;67:613–619. [Google Scholar]
- Rowe D.K., Graynoth E. Ministry for the Environment; 2002. Fish in New Zealand Lakes. [Google Scholar]
- Rowe D.K., Konui G., Christie K.D. Population structure, distribution, reproduction, diet, and relative abundance of koaro (Galaxias brevipinnis) in a New Zealand lake. J. R. Soc. New Zeal. 2002;32:275–291. [Google Scholar]
- Telfer S., Bown K.J., Sekules R., Begon M., Hayden T., Birtles R. Disruption of a host–parasite system following the introduction of an exotic host species. Parasitology. 2005;130:661–668. doi: 10.1017/s0031182005007250. [DOI] [PubMed] [Google Scholar]
- Tompkins D.M., White A.R., Boots M. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett. 2003;6:189–196. [Google Scholar]
- Tompkins D.M., Poulin R. In: Biological Invasions in New Zealand. Allen R.B., Lee W.G., editors. Springer; Berlin, Heidelberg: 2006. Parasites and biological invasions; pp. 67–86. [Google Scholar]
- Townsend C.R. Individual, population, community, and ecosystem consequences of a fish invader in New Zealand streams. Conserv. Biol. 2003;17:38–47. [Google Scholar]
- Trouve S., Sasal P., Jourdane J., Renaud F., Morand S. The evolution of life history traits in parasitic and free-living platyhelminthes: a new perspective. Oecologia. 1998;115:370–378. doi: 10.1007/s004420050530. [DOI] [PubMed] [Google Scholar]
- Van Riper C., Van Riper S.G., Hansen W.R. Epizootiology and effect of avian pox on Hawaiian forest birds. Auk. 2002;119:929–942. [Google Scholar]
- Wiles G.J., Bart J., Beck R.E., Aguon C.F. Impacts of the brown tree snake: patterns of decline and species persistence in Guam’s avifauna. Conserv. Biol. 2003;17:1350–1360. [Google Scholar]


