Graphical abstract
Keywords: Gastrointestinal helminthes, Invasive species, Nyctereutes procyonoides, Vulpes vulpes, Echinococcus multilocularis, Alaria alata, Intestinal distribution, Zoonosis
Highlights
-
•
Raccoon dogs recently invaded Denmark.
-
•
Raccoon dogs and foxes harboured several helminths, some are zoonotic.
-
•
A markedly different helminth fauna was evident between the two hosts.
-
•
A fox was infected with Echinococcus multilocularis, no Trichinella spp. were detected.
-
•
Wildlife surveillance is a foundation for the protection against communicable parasites.
Abstract
Invasive species negatively influence the biodiversity of the ecosystems they invade and may introduce pathogens to native species. Raccoon dogs have very successfully invaded Europe, including, recently, Denmark. This study included analyses of gastrointestinal helminths and Trichinella spp. from 99 raccoon dogs and 384 native red foxes collected from October 2009 to March 2012. The sedimentation and counting method used revealed that raccoon dogs and foxes harboured 9 and 13 different helminth species, respectively, of which several known to be zoonotic. Significantly more nematode and cestode species were found in foxes while raccoon dogs had more trematode species. Rodent transmitted parasites were more prevalent in foxes, while amphibian transmitted parasites were more prevalent in raccoon dogs. One fox was infected with Echinococcus multilocularis (0.3%), while no Trichinella spp. were detected in raccoon dogs or foxes. The trematode Brachylaima tokudai was detected for the first time in Denmark in five of 384 foxes (1.3%). Prevalences of Pygidiopsis summa (3.0% and 3.4%) and Cryptocotyle spp. (15.2% and 15.4%) were comparable in raccoon dogs and foxes, respectively. Four helminth species were more prevalent in foxes than in raccoon dogs: Toxocara canis (60.9% and 13.1%); Uncinaria stenocephala (84.1% and 48.5%); Mesocestoides spp. (42.7% and 23.2%); and Taenia spp. (30.7% and 2.0%), respectively. Three helminth species were more prevalent in raccoon dogs than in foxes: Dipylidium caninum (5.1% and 0.3%); Mesorchis denticulatus (38.4% and 4.2%); and Alaria alata (69.7% and 34.4%), respectively. T. canis was more abundant in foxes while A. alata was more abundant in raccoon dogs. The intestinal distribution of a number of helminth species was comparable between hosts, but highly variable between parasite species. Inherent biological factors and host invasion of new areas might have shaped these marked differences in helminth fauna between the invasive raccoon dog and the native red fox.
1. Introduction
The raccoon dog (Nyctereutes procyonoides) was deliberately introduced into the Western part of the former Soviet Union in the 1920s for use in fur production, and later migrated to numerous European countries. In Denmark, only one raccoon dog was observed prior to 1995. Throughout the period 1995–2003, 25 raccoon dogs were reported (Baagøe and Ujvári, 2007). However, in 2008 five raccoon dogs were shot or road-killed. Subsequently, and coinciding with increased public awareness, from July 2008 to December 2010, a total of 91 raccoon dogs were shot or road-killed (Danish Nature Agency, 2010), all in the peninsula of Jutland which is the only part of Denmark connected to mainland Europe, via the border to Germany.
In addition to its negative effects on biodiversity, the introduction of raccoon dogs is associated with the risk of introducing parasites to new areas (Ivanov and Semenova, 2000). Numerous field surveys on raccoon dogs have documented the presence of several parasite species of zoonotic potential (Zablotskii, 1970, Sato et al., 1999a, Sato et al., 1999b, Ivanov and Semenova, 2000, Thiess et al., 2001, Shimalov and Shimalov, 2002, Anisimova, 2008, Kornyushin et al., 2011, Bruzinskaite-Schmidhalter et al., 2012). Among the most important zoonotic parasites transmitted by raccoon dogs in Europe are nematodes of the genus Trichinella. Wild carnivores are major hosts contributing to the transmission of the infection to pigs and subsequently to humans (Malakauskas et al., 2007). A field study in Finland concluded that raccoon dogs together with red foxes were the most important reservoir hosts for Trichinella spp. (Airas et al., 2010). This is of particular interest in non-endemic countries, for example Denmark (Enemark et al., 2000), where increasing numbers of invading raccoon dogs may affect the pig industry negatively, and therefore the Trichinella prevalence is continuously monitored in wild carnivores (Danish Zoonosis Centre, 2012).
Another important zoonotic parasite of the raccoon dog is Echinococcus multilocularis. Experimental infections showed that the raccoon dog excrete infective eggs in quantities similar to that of foxes (Kapel et al., 2006). In Europe, adult E. multilocularis were recovered from naturally infected raccoon dogs in Germany (Thiess et al., 2001, Wolf and Weber, 2009), Poland (Machnicka-Rowińska et al., 2002), and Lithuania (Bruzinskaite-Schmidhalter et al., 2012). However, the role of raccoon dogs in the life cycle of E. multilocularis is still questionable because of lower prevalence and abundance within natural infection, possibly because rodents are less common in their diet than in the diet of foxes (Bruzinskaite-Schmidhalter et al., 2012). In addition, raccoon dogs are shy animals and maintain a long distance from human activities. They are also nocturnal, settle in wet habitats, and hibernate in winter (Kauhala and Kowalczyk, 2011), and these characteristics may limit their role in E. multilocularis transmission. In Denmark, E. multilocularis was detected for the first time in three of 646 foxes from the island of Zealand in 2000 (Saeed et al., 2006), but nothing is known about the current prevalence, and the present study is the first for a decade to estimate the prevalence of this important zoonotic parasite among foxes and raccoon dogs.
In 2009 the Danish Ministry of the Environment initiated a plan for eradication of raccoon dogs primarily because of the expected negative effects on biodiversity (Danish Nature Agency, 2010). This provided an opportunity to study intestinal helminths and Trichinella spp. in raccoon dogs and to compare their parasite fauna with that of the red foxes (Vulpes vulpes).
2. Materials and methods
2.1. Study area and animal sampling and processing
Animals were collected from two major regions in Denmark, the mainland and the islands. The mainland is a peninsula named Jutland and is connected to mainland Europe, whereas the islands (Zealand, Funen, Møn and Lolland) that are separated from the mainland. Both regions are relatively flat (maximum elevation above sea level: 173 m) and are rich in woodlands and agricultural areas, as well as villages, small towns and cities (Statistics Denmark, 2012). During the period October 2009–March 2012 raccoon dogs that had been road-killed, hunted or caught in traps and euthanized as part of the eradication plan in mainland were examined. Foxes that had been hunted were collected from throughout Denmark. All animals were necropsied, and animal age (young < 1 year; adult > 1 year; Kauhala and Helle, 1990), season of sampling (only given for raccoon dogs since foxes were hunted only in winter), gender, weight and general health condition were recorded.
Viscera of animals sampled were frozen for at least 4 days at −80 °C to deactivate viable eggs of E. multilocularis (Eckert et al., 2001). Subsequently, the viscera were thawed and dissected using the intestinal sedimentation and counting technique (Hofer et al., 2000). Briefly, the small intestine was divided into three equal sections. Solid pieces of intestinal contents were removed before transferring the sections into a 1 L glass bottle containing physiological saline solution. After vigorous shaking for approximately 20 s, the intestinal sections were removed after scraping the mucosa from the underlying tissue by running the intestine between two fingers. The intestinal contents were then left to sediment for at least 15 min, after which the supernatant was decanted and the bottle was refilled with saline. This washing procedure was repeated until a clear supernatant was obtained. The sediment was stored in jars with 70% ethanol for identification and counting of parasites. The entire sediment was examined in Petri dishes using a stereomicroscope at 100× magnification, and the helminths recovered were identified according to Euzeby (1982) and Christensen and Roth (1949). When helminth counts exceeded 100 before the entire sediment had been examined, one sub-sample was examined following a 20:1 dilution with physiological saline. If E. multilocularis was detected, the 12S rRNA gene was sequenced (Stefanic et al., 2004). Larvae of Trichinella spp. were detected and recovered using the magnetic stirrer method according to Commission Regulation No 2075/2005/EC (European Commission, 2005).
2.2. Statistics
Statistical analyses were performed using SAS statistical package (SAS Enterprise Guide Version 4.3®; SAS institute Inc. Cary, NC). Summaries of parasites prevalence, range of intensity of infection, mean intensity and abundance were assessed according to Bush et al. (1997), and data were presented as mean values ± 95% confidence intervals (95% CI) fitted for binomial distribution. The prevalence of parasite infections among raccoon dogs and foxes was evaluated for association with several ecological and biological factors in a negative binomial and zero inflated model. The dependent variable was the parasite infection, and the independent variables included the region sampled (two levels), season of collection (four levels), animal gender and age (two levels). Seasons were defined as follows: spring: February–April; summer: May–July: autumn: August–October; and winter: November–January. Backward and forward selection was used, and variables (p > 0.05) were removed based on Wald statistics. The difference of incidence of the different parasite groups in raccoon dogs and foxes was evaluated using the chi square statistics.
3. Results
In general, all animals were in good health and had body condition scores within normal ranges. During the study period, 99 raccoon dogs were collected only from mainland, and 384 foxes were collected throughout Denmark, although mainly from the mainland (N = 209, Table 1, Fig. 1). Complete data were not available for all animals included, for example because of decapitation and/or removal of the animal’s fur for conservation purposes (Table 1). All parasite species detected in raccoon dogs were also recovered from foxes, whereas four species were recovered from foxes only (Table 2). The parasite species richness was significantly different (p < 0.01) in foxes and raccoon dogs with averages of 2.8 and 2.2 species, respectively. In total, 11.1% of the raccoon dogs and 2.9% of the foxes were free of helminths. Of the raccoon dogs sampled 23.2% were infected with one parasite species, 26.3% with two species, 23.2% with three species, 9.1% with four species, 6.1% with five species and 1% were infected with six parasite species. In comparison, 14.8% of the sampled foxes were infected with one parasite species, 26.3% with two species, 25.3% with three species, 20.1% with four species, 8.6% with five species, 1.8% with six species and 0.3% were infected with seven parasite species. Twenty E. multilocularis were recovered from one fox (1 of 384 = 0.26%) shot close to the German border, and the finding was confirmed by sequencing of the 12S rRNA gene (Enemark and Nielsen, 2012, Enemark et al., 2013). Infections with Trichinella spp. were found in raccoon dogs or foxes.
Table 1.
Demographic information for foxes and raccoon dogs collected for parasitological examination in Denmark 2009–2012.
| Variable | Category | Animal species |
|
|---|---|---|---|
| Fox (N = 384) | Raccoon dog (N = 99) | ||
| Gender | Male | 153 (39.8%) | 48 (48.5%) |
| Female | 183 (47.7%) | 47 (47.5%) | |
| Not recorded | 48 (12.5%) | 4 (4%) | |
| Age | <1 year old | 107 (27.9%) | 29 (29.3%) |
| >1 year old | 224 (58.3%) | 55 (55.6%) | |
| Not recorded | 53 (13.8) | 15 (15.2%) | |
| Weight | <3 kg | 0 | 13 (13.1%) |
| 3–4.9 kg | 14 (3.6%) | 22 (22.2%) | |
| 5–6.9 kg | 186 (48.4%) | 29 (29.3) | |
| >7 kg | 121 (31.5%) | 20 (20.2%) | |
| Not recorded | 63 (16.4%) | 15 (15.2%) | |
| Region | Mainland | 218 (56.8%) | 99 (100%) |
| Islands | 162 (24.2%) | 0 | |
| Not recorded | 4 (1%) | 0 | |
| Season⁎ | Autumn | – | 21 (21.2%) |
| Spring | – | 10 (10.1%) | |
| Summer | – | 11 (11.1%) | |
| Winter | – | 43 (43.4%) | |
| Not recorded | – | 14 (14.1%) | |
Reported only for raccoon dogs as foxes were solely hunted during winter.
Fig. 1.
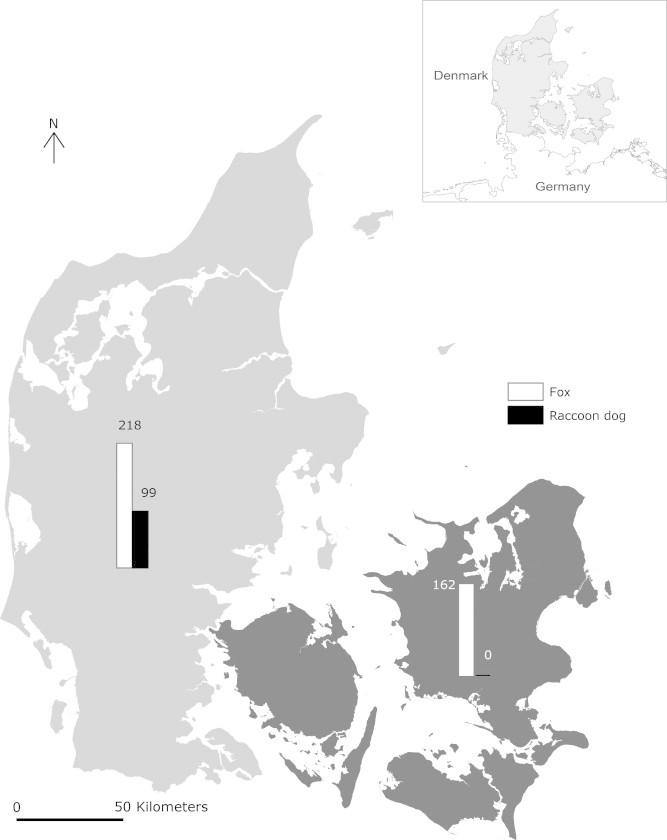
A map of Denmark showing the regions where the animals were sampled from 2009 to 2012. The grey shading indicates the mainland (Jutland), and the black shading the islands (Zealand, Funen, Møn, Lolland). Numbers above the bars are the sample sizes of each host species in each region.
Table 2.
Summary statistics of the parasites recovered from foxes and raccoon dogs. Data on range of intensity, mean intensity and abundance in foxes were available only for 314 individuals.
| Parasite group | Parasite species | Host | Prevalence (95% CI) | Range of intensity | Mean intensity (95% CI) | Abundance (95% CI) |
|---|---|---|---|---|---|---|
| Nematode | Toxocara canis⁎ | Fox | 60.9 (56–66) | 1–28 | 4.3 (3.7–5.0) | 2.6 (2.2–3.1) |
| Raccoon dog | 13.1 (7–21) | 1–5 | 1.8 (1.0–2.5) | 0.2 (0.1–0.4) | ||
| Uncinaria stenocephala⁎ | Fox | 84.1 (81–88) | 1–111 | 13.2 (11.2–15.1) | 11.2 (9.5–12.9) | |
| Raccoon dog | 48.5 (38–59) | 1–98 | 12.4 (7.5–17.2) | 6.1 (3.5–8.8) | ||
| Cestode | Mesocestoides spp.⁎ | Fox | 42.7 (38–48) | 1–250 | 41.3 (32.3–50.3) | 16.6 (12.3–20.8) |
| Raccoon dog | 23.2 (15–33) | 1–190 | 25.3 (7.4–43.3) | 6.1 (1.5–10.8) | ||
| Taenia spp.⁎ | Fox | 30.7 (26–36) | 1–83 | 7.2 (4.6–9.9) | 2.1 (1.2–2.9) | |
| Raccoon dog | 2.0 (0–7) | 1–2 | 1.5 (0–7.9) | 0.03 (0–0.1) | ||
| Dipylidium caninum⁎ | Fox | 1.0 (0–2) | 1–2 | 1.25 (0.5–2.0) | 0.02 (0–0.03) | |
| Raccoon dog | 5.1 (2–11) | 1–2544 | 367.36 (0–1255) | 26.0 (0–77.0) | ||
| Echinococcus multilocularis | Fox | 0,3 (0–1) | 20 | 20 (–) | 0.06 (0–0.2) | |
| Raccoon dog | – | – | – | – | ||
| Trematode | Pygidiopsis summa | Fox | 3.4 (2–6) | 1–189 | 26.6 (0–60.1) | 1.0 (0–2.3) |
| Raccoon dog | 3.0 (1–9) | 1–103 | 48.0 (0–118.6) | 1.7 (0–7.1) | ||
| Mesorchis denticulatus⁎ | Fox | 4.2 (2–7) | 1–26 | 4.8 (0.8–8.9) | 0.2 (0–0.4) | |
| Raccoon dog | 38.4 (29–49) | 1–68 | 13.1 (7.8–18.4) | 5.2 (2.7–7.6) | ||
| Brachylaima tokudai | Fox | 1.3 (0–3) | 1–79 | 17.2 (0–60.1) | 0.3 (0–0.8) | |
| Raccoon dog | – | – | – | – | ||
| Metorchis bilis | Fox | 0.3 (0–1) | 1 | 1 (–) | 0 (0–0.01) | |
| Raccoon dog | – | – | – | – | ||
| Cryptocotyle spp. | Fox | 15.4 (12–19) | 1–1362 | 110.3 (43.1–177.4) | 16.2 (5.7–26.6) | |
| Raccoon dog | 15.2 (9–24) | 1–5636 | 402.2 (0–1099) | 69.1 (0–182.5) | ||
| Alaria alata⁎ | Fox | 34.4 (30–39) | 1–1128 | 35.2 (12.0–58.3) | 12.2 (4.0–20.4) | |
| Raccoon dog | 69.7 (60–79) | 1–13305 | 765.4 (323.7–1207) | 533.7 (220–847) | ||
| Acanthocephala | Macracanthorhynchus catulinus | Fox | 0.5 (0–2) | 1 | 1 (–) | 0 (0–0.01) |
| Raccoon dog | – | – | – | – | ||
Zoonotic parasites are presented in bold.
Significant difference in prevalence in raccoon dogs and foxes (p<0.05).
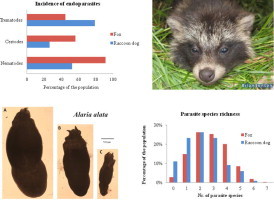
In raccoon dogs only one parasite species, Alaria alata, was present in >50% of the hosts (dominant prevalence; Esch and Fernandez, 1993), whereas two nematode species (Toxocara canis and Uncinaria stenocephala) were dominant in foxes. Five intermediate species (present in 10–50% of the hosts) were found in raccoon dogs: Cryptocotyle spp.; Mesorchis denticulatus; Mesocestoides spp.; T. canis; and U. stenocephala, and four intermediate species were found in foxes: A. alata; Cryptocotyle spp.; Taenia spp.; and Mesocestoides spp. The remaining satellite species (present in <10% of the hosts) in raccoon dogs were: Pygidiopsis summa; Dipylidium caninum; and Taenia spp., and in foxes: D. caninum; E. multilocularis; P. summa; M. denticulatus; Brachylaima tokudai; and Metorchis bilis. B. tokudai was isolated from five foxes (1.3%).
Prevalence and abundance of two trematodes (P. summa and Cryptocotyle spp.) were comparable between the hosts (Table 2). However, for two nematode species (T. canis and U. stenocephala) and two cestode genera (Mesocestoides spp. and Taenia spp.) prevalence and abundance were significantly higher in foxes, whereas the prevalence and abundance of one cestode (D. caninum) and one trematode (M. denticulatus) were significantly higher in raccoon dogs. For all parasite groups there were significant differences between hosts (p < 0.01) in the frequency of parasites recovered (Table 3).
Table 3.
Incidence rate (95% CI) of parasite groups recovered from raccoon dogs (N = 99) and foxes (N = 384).
| Animal species | Nematodes⁎ | Cestodes⁎ | Trematodes⁎ | Acanthocephala | Total⁎ |
|---|---|---|---|---|---|
| Raccoon dog | 52.5 (42.2–62.7) | 26.3 (17.9–36.1) | 78.8 (69.4–86.4) | 0 | 88.9 (81–94.3) |
| Fox | 91.2 (87.9–93.8) | 56.3 (51.1–61.3) | 44.5 (39.5–49.7) | 0.5 (0.1–1.9) | 97.1 (94.9–98.6) |
Significant difference (p < 0.01) in the frequency rate calculated by chi square statistics is present between host species.
The prevalence of three helminth species; T. canis; U. stenocephala; and Taenia spp., were significantly lower in raccoon dogs than in foxes in both regions. On the other hand the prevalence of A. alata and M. denticulatus were significantly higher in raccoon dogs compared to foxes from both regions. The prevalence of Mesocestoides spp. was lower in raccoon dogs than in foxes in mainland but not in the islands. No significant differences in the prevalences of P. summa and Cryptocotyle spp. were detected between raccoon dogs and foxes.
The distributions of a number of helminth species within the intestine were comparable in raccoon dogs and foxes, but were highly variable between parasite species. For example, more than 50% of T. canis, A. alata and Cryptocotyle spp. were recovered from the anterior segment of the small intestine in both hosts, whereas the same trend was observed for Taenia spp. in foxes only and for D. caninum in raccoon dogs only. On the other hand, less than 20% of U. stenocephala and Mesocestoides spp. were recovered from the anterior intestinal segment of both hosts, and the same trend was observed for P. summa and M. denticulatus in foxes only. The posterior intestinal segment harbored in general the lowest worm infra-populations for many helminth species in both hosts, except for U. stenocephala in both hosts and M. denticulatus and P. summa in foxes.
All parasite species were of similar morphology in both hosts with the exception of A. alata. We noticed three size classes of this parasite: large (approximately 3.6 mm in length); medium (approximately 2.7 mm); and small (approximately 1.8 mm). In raccoon dogs, 92.3% of all recovered A. alata were classified as small and were very abundant, with a maximum count of 13,305 in one host. The remaining A. alata from infected raccoon dogs were classified as medium. In foxes 70.5% of all recovered A. alata were classified as large and were less abundant, with a maximum count of 215 in one host. Medium sized A. alata were recovered from 17.1% of all infected foxes, and the abundance similar to that of the large flukes. The small A. alata were recovered from the reminder of the infected foxes (12.4%) with a maximum count of 1128 in one host.
The multivariate analyses showed no significant interactions between risk factors for parasites species in raccoon dogs and foxes sampled, but risk factors were individually associated with the abundance of a number of parasite species. Positive regression parameters (Table 4) indicates increased risk of infection with a certain parasite species due to the presence of a specific risk factor in comparison to a reference factor (winter for season, juvenile for age, male for gender and mainland for region), while the negative regression parameter indicates the opposite situation. The incidence rate ratio for each parasite species indicates the relative abundance of that parasite when affected by a given risk factor, and is presented first and the rest of the additive incidence ratios for each factor are given later. For example, the expected abundance of Mesocestoides spp. in raccoon dogs in relation to seasons (1.77) is increased in spring by a factor of 5.43 but only increased by 0.15 in summer.
Table 4.
Multivariate analysis of risk factors related to increased abundance of parasite infections in raccoon dogs and foxes in Denmark. Abundance was calculated according to the likelihood profile.
| Host | Parasite | Factor | Regression parameter (standard error) | Incident rate ratio (95% CI) |
|---|---|---|---|---|
| Raccoon dogs | Mesocestoides spp. | 0.57 (0.58) | 1.77 (5.49–0.57) | |
| Season | Spring | 1.69 (1.31) | 5.43 (71.34–0.41) | |
| Summer | −1.87 (1.39) | 0.15 (2.34–0.01) | ||
| Autumn | 2.05 (1.00) | 7.73 (54.67–1.09) | ||
| Winter | ||||
| Alaria alata | 6.67 (0.38) | 789.66 (1676.78–371.88) | ||
| Season | Spring | −1.37 (0.88) | 0.25 (1.44–0.04) | |
| Summer | −2.69 (0.85) | 0.07 (0.36–0.01) | ||
| Autumn | −0.23 (0.67) | 0.79 (2.96–0.21) | ||
| Winter | ||||
| Cryptocotyle spp. | ||||
| Age | Adult | 4.02 (1.51) | 55.48 (1067.16–2.88) | |
| Juvenile | ||||
| Season | Spring | −0.13 (2.14) | 0.87 (57.82–0.01) | |
| Summer | 5.85 (2.41) | 347.10 (38989.36–3.09) | ||
| Autumn | 2.98 (1.51) | 19.72 (380.47–1.02) | ||
| Winter | ||||
| Fox | Toxocara canis | 0.77 (0.13) | 2.17 (2.81–1.68) | |
| Gender | Female | −0.58 (0.18) | 0.56 (0.79–0.39) | |
| Male | ||||
| Region | Islands | 0.70 (0.18) | 2.01 (2.85–1.42) | |
| Mainland | ||||
| Uncinaria stenocephala | 2.14 (0.14) | 8.51 (11.14–6.50) | ||
| Age | Adult | 0.37 (0.17) | 1.45 (2.01–1.05) | |
| Juvenile | ||||
| Alaria alata | 0.56 (0.42) | 1.75 (3.99–0.76) | ||
| Region | Islands | 1.14 (0.42) | 3.14 (7.15–1.38) | |
| Mainland | ||||
| Age | Adult | 0.87 (0.44) | 2.38 (5.61–1.01) | |
| Juvenile | ||||
| Cryptocotyle spp. | 3.14 (0.50) | 23.07 (61.73–8.62) | ||
| Gender | Female | −1.60 (0.75) | 0.20 (0.88–0.05) | |
| Male | ||||
| Mesorchis denticulatus | −3.03 (0.56) | 0.05 (0.14–0.02) | ||
| Region | Islands | 2.37 (0.83) | 10.73 (54.88–2.10) | |
| Mainland | ||||
| Pygidiopsis summa | −4.54 (1.41) | 0.01 (0.17–0.00) | ||
| Age | Adult | 4.91 (1.57) | 135.88 (2957.03–6.24) | |
| Juvenile | ||||
In summary, the multivariate analysis of data on raccoon dog parasites revealed different season-associated abundance of Mesocestoides spp., A. alata and Cryptocotyle spp., while the abundance of Cryptocotyle spp. was also associated with the age of host (Table 4). Regression parameter revealed reduced incidence of Mesocestoides spp., in summer and winter compared to spring and autumn, increased incidence of A. alata in winter compared to other seasons, and reduced incidence of Cryptocotyle spp. in spring and winter compared to summer and autumn. The incidence of Cryptocotyle spp. in adult raccoon dogs was higher than in juvenile ones. In foxes, six parasite species were associated with one or two risk factors (Table 4). Regression parameters revealed increased incidence of U. stenocephala, A. alata and P. summa in adult foxes compared to juveniles. The incidence of T. canis, A. alata and M. denticulatus was higher in mainland than in islands. Finally, the incidence of T. canis and Cryptocotyle spp. was higher in male foxes than in females.
4. Discussion
Previous epidemiological studies of natural infections in raccoon dogs in Europe showed that a wide range of parasites can infect this host (Zablotskii, 1970, Sato et al., 1999a, Sato et al., 1999b, Ivanov and Semenova, 2000, Thiess et al., 2001, Shimalov and Shimalov, 2002, Anisimova, 2008, Kornyushin et al., 2011, Bruzinskaite-Schmidhalter et al., 2012). Despite the enormous variation in the location of previous studies in terms of biotic and abiotic conditions, the invading raccoon dog in Europe has established a parasite fauna that is very similar to the closely related carnivore, the red fox (Ivanov and Semenova, 2000, Thiess et al., 2001, Bruzinskaite-Schmidhalter et al., 2012). Of the raccoon dogs examined in the present study, 11.1% were free of helminths. This contrasts with a survey in the Volga Delta, where raccoon dogs had been established for 30 years, which found parasites in all of 80 animals examined (Zablotskii, 1970). The reduced parasite biodiversity in invading hosts compared to native hosts is an expected consequence of invasion (Torchin et al., 2003).
The most prevalent and abundant parasite of raccoon dogs in Europe is A. alata with prevalences ranging from 23.5% (Ivanov and Semenova, 2000) to 96.5% (Bruzinskaite-Schmidhalter et al., 2012). The abundance of A. alata among raccoon dogs in Europe ranged from 13 (Ivanov and Semenova, 2000) to 5694 with as many as 660,396 worms recovered from a single animal (Anisimova, 2008). Such highly abundant parasite species may have benefited from less competition with other species (Roche et al., 2010), or the abundance may reflect higher levels of host susceptibility.
Four parasite species were detected in foxes but not in raccoon dogs: M. catulinus; E. multilocularis; B. tokudai; and M. bilis; of these, only E. multilocularis has been recorded previously in foxes in Denmark. The other three have been found as satellite species in foxes in the Iberian Peninsula (Segovia et al., 2002, Segovia et al., 2004). B. tokudai has not been reported previously in raccoon dogs in Europe, but is prevalent in this host in Japan (Sato et al., 1999b). M. bilis was previously diagnosed in foxes (Schuster et al., 2003) and cats (Schuster et al., 1997) in Germany. It should be noted, however, that differences between raccoon dogs and foxes with respect to species abundance may have been affected by the greater number of foxes included in the present study. Likewise, the use of the sensitive sedimentation and counting technique to isolate and detect intestinal helminths (Deplazes et al., 2004) may lead to the detection of new parasite species, especially those that are very small, and may have increased the sensitivity of the present study.
Despite similar parasite species richness in raccoon dogs and foxes marked differences in prevalence and abundance of helminths were evident in the present study. Earlier reports from Denmark showed that D. caninum and M. denticulatus are uncommon among foxes (Saeed et al., 2006) but common in dogs (Christensen and Roth, 1949), which may have been sources of infection for the raccoon dogs. The current results also showed comparable prevalences of two trematodes, P. summa and Cryptocotyle spp., in foxes and raccoon dogs. Cryptocotyle spp. has a broad host spectrum with a range of fish-eating birds and mammals as potential hosts, and is moderately pathogenic (Christensen and Roth, 1949). In Denmark, M. denticulatus and Cryptocotyle spp. were previously reported from naturally infected dogs (Christensen and Roth, 1949). P. summa, on the other hand, present in foxes in this study, has never before been reported from raccoon dogs in Europe although this parasite was detected in this host in Japan (Sato et al., 1999b).
In raccoon dogs, the abundance of three parasite species was affected by season of sampling: Mesocestoides spp.; A. alata; and Cryptocotyle spp., and the later species was also affected by age of host. The marked variation in the seasonal incidence of these parasites may be related to variations on the availability of intermediate hosts (Mesocestoides spp. is transmitted by feeding on rodents, A. alata by amphibians and Cryptocotyle spp. by fish) or a reflection of increased hunting pressure on raccoon dogs that force them not to hibernate in winter, but to change its location continuously and feed on whatever available in nature. In a similar study in raccoon dogs in Lithuania (Bruzinskaite-Schmidhalter et al., 2012), the abundance of A. alata and U. stenocephala was affected by season, in which abundance was markedly reduced in winter due to hibernation. In foxes, the abundance of several parasite species was associated with one or more risk factors. For example, the abundance of three parasite species was affected by the region, the abundance of three parasite species was affected by age and finally the abundance of two parasite species was affected by gender. Region-associated differences in the parasite abundance could be a result of local biotic conditions, thus further investigations of the underlying mechanisms are warranted. Differences in abundance associated with gender and age might be related to behavior, age acquired immunity or life expectancy of parasites (Bruzinskaite-Schmidhalter et al., 2012).
A comparison between the helminth diversity and prevalence in raccoon dogs and foxes enables an assessment of host exposure and susceptibility. Contrary to the situation in foxes, the dominant parasite species recovered from raccoon dogs in the current study was an amphibian-transmitted trematode, whereas the dominant species recovered from foxes were two nematodes: T. canis; and U. stenocephala, both of which have a direct life cycle with the possibility of a rodent paratenic host (Taylor et al., 2007). Sharing the dens between raccoon dogs and other animals, mainly foxes and badgers (Kowalczyk et al., 2008), may increase the risk of infection with these species and with other parasites with direct life cycles. Also, higher prevalence and/or abundance of one cestode species (D. caninum) and two trematode species (M. denticulatus and A. alata) in raccoon dogs compared to foxes were recorded in the present study. Such differences might be explained by different host susceptibility, food preferences (Thiess et al., 2001), and/or food availability. The diet of raccoon dogs varies with season and location, and generally small mammals, insects and plants constitute the major part of the diet both in native and non-native areas (Sutor et al., 2010). Furthermore, raccoon dogs have a special defecating behavior that might contribute to a different spatial transmission of parasites. Raccoon dogs tend to concentrate their faeces in confined areas (called latrines) (Yamamoto and Hidaka, 1984), whereas foxes defecate in a dispersed or scattered fashion. This difference might affect parasites with density-dependant transmission (Torchin and Mitchell, 2004). On the other hand, proximity of foxes to water bodies and the abundance of amphibians and snails were among the crucial factors that led to a higher prevalence of trematodes (Eira et al., 2006, Saeed et al., 2006). The higher prevalence of Mesocestoides spp. and Taenia spp. in foxes reported in the present study can probably be explained by higher predation of foxes on rodent intermediate hosts of these parasites (Bruzinskaite-Schmidhalter et al., 2012).
The current report showed marked differences in parasite prevalences in foxes when compared to a previous report by Saeed et al. (2006). For example, in the current study, prevalences of T. canis, U. stenocephala, Taenia spp. and A. alata in the islands are significantly higher than those previously reported from the same region (Saeed et al., 2006). These changes in prevalences may have resulted from using different techniques of animal sampling and/or worm isolation, or because of other concurrent factors, such as drastic changes in the fox population cause by the epidemic scabies in Zealand (Forchhammer and Asferg, 2000). However, when comparing the two data sets we could not determine whether the change in the helminth fauna of foxes on the mainland was induced by the invasion of raccoon dogs, especially since in this region these are highly dispersed, very limited in numbers and under intense hunting pressure because of the eradication program.
When comparing the current results to those recently reported from Lithuania (Bruzinskaite-Schmidhalter et al., 2012), we found that in both countries the most prevalent parasites of raccoon dogs were A. alata and U. stenocephala, and for both parasites the prevalence was significantly higher in Lithuania than in Denmark. Contrary to this, the prevalence of M. denticulatus/Echinostomatidea in raccoon dogs was significantly higher in Denmark than in Lithuania, and in both countries the prevalence was higher in raccoon dogs than in foxes. The prevalences of T. canis and Mesocestoides spp. in raccoon dogs in both countries were comparable and were relatively low, whereas foxes from both countries had higher prevalences of T. canis; and Mesocestoides spp. than raccoon dogs. The prevalence of A. alata in Lithuania was equally high for both hosts, but was higher in raccoon dogs than in Danish foxes. Raccoon dogs were relatively newly introduced to Denmark; hence, the relatively low prevalences of helminths in comparison to Lithuania may reflect reduced exposure periods to parasites, and the differences in parasite prevalences in the fox. Nonetheless, these results may suggest higher susceptibility of raccoon dogs to certain parasite species, especially trematodes.
In this study, 10 of the 13 parasite species recovered from foxes and raccoon dogs were of zoonotic importance. The most important among these was E. multilocularis which was recovered from only one fox and was reported previously (Enemark and Nielsen, 2012). In 2000 this parasite was detected in foxes for the first time in Denmark, in the island of Zealand (Kapel and Saeed, 2000). Prior to the present study, however, E. multilocularis had not been detected in any other region of Denmark. The sample size and the low prevalence made it impossible to detect differences in the geographic distribution and prevalence of E. multilocularis in Denmark. Likewise, the sample size did not allow detection of changes in the prevalence of E. multilocularis in Zealand since 2000. However, the location of the infected fox approximately 8 km north of the German border may suggest introduction and transmission by foxes migrating from endemic areas south of Denmark. The presence of Trichinella spp. in wild carnivores has been monitored in Denmark for several years as a part of the risk-based monitoring program required by European Union member states with a negligible risk of Trichinella (European Commission, 2005), and the prevalence of Trichinella spp. has been continuously low (Enemark et al., 2000, Alban et al., 2011). Thus, although both foxes and raccoon dogs are considered suitable indicator hosts (Oivanen et al., 2002), the lack of Trichinella-positive animals in the present study was anticipated. The currently observed higher mean abundance of A. alata, D. caninum, Cryptocotyle spp., and M. denticulatus, in raccoon dogs in the current study may indicate a possible contribution of that host to increase environmental contamination with potentially zoonotic agents through parasite spillback (Kelly et al., 2009). However, as the invading population of raccoon dogs in Denmark is limited at its current low level, a significant effect on native hosts is not anticipated.
The present study showed different parasite distribution in the intestinal tract within one host but comparable between hosts. Habitat selection by parasites might be a natural response to external factors including inter-specific competition for nutrients, space, or other critical resources between dominant and less dominant species (Poulin, 1999). Although competition between helminth species for the anterior small intestine may be driven by the optimal nature of that location (Sukhdeo, 1991, El-Shehabi et al., 1999), site selection by helminths is rather dynamic (Poulin, 1999). This was demonstrated experimentally when the cestode Hymenolepis diminuta migrated along the intestine in response to intestinal contraction that accompanied the passage of food (Sukhdeo, 1992). Furthermore, even in the absence of competitors and the availability of an optimal site, Trichinella spiralis selected a sub-optimal niche that resulted in reduced fecundity of its females (Sukhdeo, 1991). Different parasite species exhibit different response to external stimuli, hence it is difficult to explain site selection in the intestine and any possible interactions between different parasite species (Sukhdeo and Bansemir, 1996).
In this study, the morphology of A. alata was highly variable in raccoon dogs and foxes. Pence et al. (2003) observed two morphological classes of A. marcianae recovered from Ocelots in USA, and suggested that it may correspond to different helminth generations, repeated infections with younger helminths, a result of helminth crowding, or caused by infection with different metacercaria strains. In trematodes, higher intensity of infection in the intermediate hosts resulted in smaller sized larvae (Saldanha et al., 2009) that may subsequently result in reduced adult sizes (Poulin and Latham, 2003). In a study involving the trematode Schistosoma mansoni, significant morphological changes were evident in the adults after one passage from naturally infected rodents to experimentally infected laboratory mice (Neves et al., 2004). Host-induced changes in the morphology of trematodes were also observed when different hosts were infected with the same parasite isolate, which caused reduced reproductive output in smaller worms (Watson and Pike, 1993). However, the significantly higher abundance of A. alata in raccoon dogs reported in the current study and other studies in Europe (Anisimova, 2008, Bruzinskaite-Schmidhalter et al., 2012) may suggest higher susceptibility of that host. Probably A. alata in raccoon dogs implemented different survival strategies to compensate for its stunted size, but further investigations are required.
5. Conclusions
Despite a relatively short period since its invasion into Denmark, raccoon dogs examined harbored a broad range of parasites, of which several are of zoonotic importance. The minute P. summa detected in the present study have never before been found in raccoon dogs in Europe or in foxes in Denmark. The use of the sensitive sedimentation and counting technique may have contributed to a better assessment of the helminth infections compared to previous studies.
The recovery of E. multilocularis from a single fox in Jutland reflects the geographical spread of this zoonotic parasite, as has been reported elsewhere in Europe, and confirms the need for continuous surveillance. Furthermore, the present study has shown the value of surveillance in the protection of people, livestock and pets from newly introduced pathogens. Rodent-transmitted parasites were more prevalent in foxes, while amphibian-transmitted species were more prevalent in raccoon dogs, and this difference may help to explain why raccoon dogs are probably less important than foxes in the transmission of E. multilocularis in Denmark.
Acknowledgements
The Danish Nature Agency, the Danish Hunters’ Association, the Danish Ministry of the Environment and Aarhus University are acknowledged for collecting and sending raccoon dogs and foxes for analysis, and employees at the National Veterinary Institute are thanked for sample handling and processing. Birgit Kristensen generated the map in Fig. 1. This Project was conducted as part of the surveillance program against infections with E. multilocularis in carnivores in Denmark, supported by the Danish Veterinary and Food Administration and the program of eradicating raccoon dogs in Denmark, supported by the Danish Ministry of the Environment.
References
- Airas N., Saari S., Mikkonen T., Virtala A.M., Pellikka J., Oksanen A., Isomursu M., Kilpelä S.S., Lim C.W., Sukura A. Sylvatic Trichinella spp. infection in Finland. J. Parasitol. 2010;96:67–76. doi: 10.1645/GE-2202.1. [DOI] [PubMed] [Google Scholar]
- Alban L., Pozio E., Boes J., Boireau P., Boué F., Claes M., Cook A.J., Dorny P., Enemark H.L., van der Giessen J., Hunt K.R., Howell M., Kirjusina M., Nöckler K., Rossi P., Smith G.C., Snow L., Taylor M.A., Theodoropoulos G., Vallée I., Viera-Pinto M.M., Zimmer I.A. Towards a standardised surveillance for Trichinella in the European Union. Prev. Vet. Med. 2011;99:148–160. doi: 10.1016/j.prevetmed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Anisimova E.I. The structure of helminths fauna of raccoon dog (Nectereutes procyonoides, Gray) nationalized in Belarus. Institute of Zoology. no. 2008;2:80–82. (in Belarusian) [Google Scholar]
- Baagøe H., Ujvári M. In: Danish Mammal Atlas. Baagøe H.J., Jensen T.S., editors. Gyldendal; Copenhagen: 2007. Raccoon dog, Nyctereutes procyonoides (Gray, 1834) pp. 182–183. (in Danish) [Google Scholar]
- Bruzinskaite-Schmidhalter R., Sarkunas M., Malakauskas A., Mathis A., Torgerson P.R., Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:418. doi: 10.1017/S0031182011001715. [DOI] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: Margolis et al., revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Christensen N.O., Roth H. Investigations on internal parasites of dogs. Aarskr Den Konglige Veterinaer - og Landbohojskole. (København) 1949;1949:1–73. [Google Scholar]
- Danish Nature Agency, 2010. The framework for action against the raccoon dog in Denmark. Ministry of Environment, Forest and Nature Agency. Mirdinec (NAT SE 344).
- Danish Zoonosis Centre . Technical University of Denmark, National Food Institute; 2012. Annual report on zoonoses in Denmark 2011. [Google Scholar]
- Deplazes P., Hegglin D., Gloor S., Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Eckert J., Gemmell M.A., Meslin F.-X., Pawlowski Z.S. World Organisation for Animal Health; Paris: 2001. WHO/OIE manual on Echinococcosis in humans and animals: a public health problem of global concern. [Google Scholar]
- Eira C., Viganda J., Torres J., Miquel J. The helminth community of the red fox, Vulpes vulpes, in Dunas de Mira (Portugal) and its effect on host condition. Wildl. Biol. Pract. 2006;2:26–36. [Google Scholar]
- El-Shehabi F.S., Abdel-Hafez S.K., Kamhawi S.A. Prevalence of intestinal helminths of dogs and foxes from Jordan. Parasitol. Res. 1999;85:928–934. doi: 10.1007/s004360050660. [DOI] [PubMed] [Google Scholar]
- Enemark, H.L., Nielsen, H.V., 2012. Fox tapeworm in Denmark: new status. EPI-NEWS. Statens Serum Institut. No 19.
- Enemark H.L., Bjørn H., Henriksen S.A., Nielsen B. Screening for infection of Trichinella in red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2000;88:229–237. doi: 10.1016/s0304-4017(99)00219-8. [DOI] [PubMed] [Google Scholar]
- Enemark, H.L., Al-Sabi, M.N.S., Knapp, J., Staahl, M., Chríel, M., 2013. Detection of a high-endemic focus of Echinococcus multilocularis in red foxes in southern Denmark, January 2013. Euro Surveill. 18 (10) pii=20420. Available online: <http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20420>. [DOI] [PubMed]
- Esch G.W., Fernandez C. Chapman and Hall; London: 1993. A functional biology of parasitism. pp. 337. [Google Scholar]
- European Commission Regulation EC No. 2075/2005 of the European Parliament and of the Council of 5 December 2005 laying down specific rules on official controls for Trichinella in meat. Off. J. EC L. 2005;338:60–82. [Google Scholar]
- Euzeby J. vol. 2. Ministry of agriculture; Paris, France: 1982. (Experimental Diagnosis of Animal Helminthiases (Domestic Animals – Laboratory Animals – Primates). Practical Work in Veterinary Helminthology). pp. 99–113. [Google Scholar]
- Forchhammer M.C., Asferg T. Invading parasites cause a structural shift in red fox dynamics. Proc. R. Soc. Lond. 2000;267:779–786. doi: 10.1098/rspb.2000.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S., Gloor S., Muller U., Mathis A., Hegglin D., Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology. 2000;120:135–142. doi: 10.1017/s0031182099005351. [DOI] [PubMed] [Google Scholar]
- Ivanov V.M., Semenova N.N. Parasitological consequences of animal introduction. Russ. J. Ecol. 2000;31:281–283. [Google Scholar]
- Kapel C.M.O., Saeed I. Echinococcus multilocularis, a new zoonotic parasite in Denmark. Dansk Vet. Tidsskr. 2000;83:14–16. (in Danish) [Google Scholar]
- Kapel C.M.O., Torgerson P.R., Thompson R.C.A., Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Kauhala K., Helle E. Age determination of the raccoon dog in Finland. Acta Theriol. 1990;35:321–329. [Google Scholar]
- Kauhala K., Kowalczyk R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: history of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011;57:584–598. doi: 10.1093/czoolo/57.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D.W., Paterson R.A., Townsend C.R., Poulin R., Tompkins D.M. Parasite spillback: a neglected concept in invasion ecology? Ecology. 2009;90:2047–2056. doi: 10.1890/08-1085.1. [DOI] [PubMed] [Google Scholar]
- Kornyushin V.V., Malayshko E.I., Malega A.M. The helminths of wild predatory mammals of Ukraine. Cestodes. Vestn. Zool. 2011;45:1–8. [Google Scholar]
- Kowalczyk R., Jędrzejewska B., Zalewski A., Jędrzejewski W. Facilitative interactions between the Eurasian badger Meles meles, the red fox Vulpes vulpes and the invasive raccoon dog Nyctereutes procyonoides in Białowieża Primeval Forest, Poland. Can. J. Zool. 2008;86:1389–1396. [Google Scholar]
- Machnicka-Rowińska B., Rocki B., Dziemian E., Kołodziej-Sobocińska M. Raccoon dog (Nyctereutes procyonoides) – the new host of Echinococcus multilocularis in Poland. Wiad Parazytol. 2002;48:65–68. [PubMed] [Google Scholar]
- Malakauskas A., Paulauskas V., Jarvis T., Keidans P., Eddi C., Kapel C.M.O. Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia and Estonia. Parasitol. Res. 2007;100:687–693. doi: 10.1007/s00436-006-0320-y. [DOI] [PubMed] [Google Scholar]
- Neves R.H., Costa-Silva M., Martinez E.M., Branquinho T.B., Oliveira R.M.F., Lenzi H.L., Gomes D.C., Machado-Silva J.R. Phenotypic plasticity in adult worms of Schistosoma mansoni (Trematoda: Schistosomatidae) evidenced by bright-field and confocal laser scanning microscopy. Mem. Inst. Oswaldo Cruz. 2004;99:131–136. doi: 10.1590/s0074-02762004000200003. [DOI] [PubMed] [Google Scholar]
- Oivanen L., Kapel C.M.O., Pozio E., La Rosa G., Mikkonen T., Sukura A. Associations between Trichinella species and host species in Finland. J. Parasitol. 2002;88:84–88. doi: 10.1645/0022-3395(2002)088[0084:ABTSAH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pence D.B., Tewes M.E., Laack L.L. Helminths of the Ocelot from Southern Texas. J. Wildl. Dis. 2003;39:683–689. doi: 10.7589/0090-3558-39.3.683. [DOI] [PubMed] [Google Scholar]
- Poulin R. The functional importance of parasites in animal communities: many roles at many levels? Int. J. Parasitol. 1999;29:903–914. doi: 10.1016/s0020-7519(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Poulin R., Latham A.D.M. Effects of initial (larval) size and host body temperature on growth in trematodes. Can. J. Zool. 2003;81:574–581. [Google Scholar]
- Roche D.G., Leung B., Franco E.F., Torchin M.E. Higher parasite richness, abundance and impact in native versus introduced cichlid fishes. Int. J. Parasitol. 2010;40:1525–1530. doi: 10.1016/j.ijpara.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Saldanha I., Leung T.L.F., Poulin R. Causes of intraspecific variation in body size among trematode metacercariae. J. Helminthol. 2009;83:289–293. doi: 10.1017/S0022149X09224175. [DOI] [PubMed] [Google Scholar]
- Sato H., Inaba T., Ihama Y., Kamiya H. Parasitological survey on wild carnivora in north-western Tohoku, Japan. J. Vet. Med. Sci. 1999;61:1023–1026. doi: 10.1292/jvms.61.1023. [DOI] [PubMed] [Google Scholar]
- Sato H., Ihama Y., Inaba T., Yagisawa M., Kamiya H. Helminth fauna of carnivores distributed in north-western Tohoku, Japan, with special reference to Mesocestoides paucitesticulus and Brachylaima tokudai. J. Vet. Med. Sci. 1999;61:1339–1342. doi: 10.1292/jvms.61.1339. [DOI] [PubMed] [Google Scholar]
- Schuster R., Kaufmann A., Hering S. Investigations on the endoparasite fauna of the domestic cat in eastern Brandenburg. Berl. Munch. Tierarztl. Wochenschr. 1997;110:48–50. [PubMed] [Google Scholar]
- Schuster R., Gregor B., Heidrich J., Nockler K., Kyule M., Wittstatt U. A sero-epidemiological survey on the occurrence of opisthorchiid liver flukes in red foxes (Vulpes vulpes) in Berlin, Germany. Parasitol. Res. 2003;90:400–404. doi: 10.1007/s00436-003-0868-8. [DOI] [PubMed] [Google Scholar]
- Segovia J.M., Torres J., Miquel J. The red fox, Vulpes vulpes L., as a potential reservoir of zoonotic flukes in the Iberian Peninsula. Acta Parasitol. 2002;47:163–166. [Google Scholar]
- Segovia J.M., Torres J., Miquel J. Helminth parasites of the red fox (Vulpes vulpes L., 1758) in the Iberian Peninsula: an ecological study. Acta Parasitol. 2004;49:67–79. [Google Scholar]
- Shimalov V.V., Shimalov V.T. Helminth fauna of the raccoon dog (Nyctereutes procyonoides Gray, 1834) in Belorussian Polesie. Parasitol. Res. 2002;88:944–945. doi: 10.1007/s00436-001-0582-3. [DOI] [PubMed] [Google Scholar]
- Statistics Denmark, 2012. Danmarks Statistikbank. www.statistikbanken.dk. Retrieved 06-August-2012.
- Stefanic S., Shaikenov B.S., Deplazes P., Dinkel A., Torgerson P.R., Mathis A. Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol. Res. 2004;92:347–351. doi: 10.1007/s00436-003-1043-y. [DOI] [PubMed] [Google Scholar]
- Sukhdeo M.V.K. The relationship between intestinal location and fecundity in adult Trichinella spiralis. Int. J. Parasitol. 1991;21:855–858. doi: 10.1016/0020-7519(91)90154-y. [DOI] [PubMed] [Google Scholar]
- Sukhdeo M.V.K. Intestinal contractions and migration behaviour in Hymenolepis diminuta. Int. J. Parasitol. 1992;22:813–817. doi: 10.1016/0020-7519(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Sukhdeo M.V.K., Bansemir A.D. Critical resources that influence habitat selection decisions by gastrointestinal helminth parasites. Int. J. Parasitol. 1996;26:483–498. doi: 10.1016/0020-7519(96)89378-7. [DOI] [PubMed] [Google Scholar]
- Sutor A., Kauhala K., Ansorge H. Diet of the raccoon dog Nyctereutes procyonoides – a canid with an opportunistic foraging strategy. Acta Theriol. 2010;55:165–176. [Google Scholar]
- Taylor M.A., Coop R.L., Wall R.L. third ed. Blackwell Publishing; Oxford, UK: 2007. Veterinary Parasitology. [Google Scholar]
- Thiess A., Schuster R., Nöckler K., Mix H. Helminth findings in indigenous raccoon dogs Nyctereutes procyonoides (Gray, 1843) Berl. Munch. Tierarztl. Wochenschr. 2001;114:273–276. [PubMed] [Google Scholar]
- Torchin M.E., Mitchell C.E. Parasites, pathogens, and invasions by plants and animals. Front. Ecol. Environ. 2004;2:183–190. [Google Scholar]
- Torchin M.E., Lafferty K.D., Dobson A.P., McKenzie V.J., Kuris A.M. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Watson J.J., Pike A.W. Variation in the morphology of adult Apatemon gracilis Rudolphi, 1819 (Digenea: Strigeidae) reared in different avian hosts. Syst. Parasitol. 1993;26:33–38. [Google Scholar]
- Wolf P., Weber M. The occurrence of Echinococcus multilocularis in foxes and raccoon dogs in Mecklenburg-Vorpommern in 2005–2007. Beitr. Jagd. Wildforsch. 2009;34:257–262. (in German) [Google Scholar]
- Yamamoto I., Hidaka T. Utilization of “latrines” in the raccoon dog, Nyctereutes procyonoides. Acta Zool. Fennica. 1984;171:241–242. [Google Scholar]
- Zablotskii V.I. The helminth fauna of the raccoon-dog acclimatised in the Volga Delta. Trudy Astrakhanskogo Zapovednika. 1970;13:364–381. [Google Scholar]


