Graphical abstract
Highlights
► Toxoplasma seroprevalences in the marsupial species examined were significantly higher where cat density was higher. ► Toxoplasma seroprevalence was significantly higher in road kill animals than culled animals. ► Higher order carnivores had significantly higher seroprevalence than medium sized browser species. ► The highest seroprevalences observed in an intermediate host was 71% in spotted-tailed quolls (Dasyurus maculatus) in areas of low cat density.
Keywords: Toxoplasma, T. gondii, Feral cat, Felis catus, Australia, Mesopredator release, Disease ecology, Tasmanian devil
Abstract
Changing ecosystem dynamics are increasing the threat of disease epidemics arising in wildlife populations. Several recent disease outbreaks have highlighted the critical need for understanding pathogen dynamics, including the role host densities play in disease transmission. In Australia, introduced feral cats are of immense concern because of the risk they pose to native wildlife through predation and competition. They are also the only known definitive host of the coccidian parasite, Toxoplasma gondii, the population-level impacts of which are unknown in any species. Australia’s native wildlife have not evolved in the presence of cats or their parasites, and feral cats may be linked with several native mammal declines and extinctions. In Tasmania there is emerging evidence that feral cat populations are increasing following wide-ranging and extensive declines in the apex predator, the Tasmanian devil, from a consistently fatal transmissible cancer.
We assess whether feral cat density is associated with the seroprevalence of T. gondii in native wildlife to determine whether an increasing population of feral cats may correspond to an increased level of risk to naive native intermediate hosts. We found evidence that seroprevalence of T. gondii in Tasmanian pademelons was lower in the north-west of Tasmania than in the north-east and central regions where cat density was higher. Also, samples obtained from road-killed animals had significantly higher seroprevalence of T. gondii than those from culled individuals, suggesting there may be behavioural differences associated with infection. In addition, seroprevalence in different trophic levels was assessed to determine whether position in the food-web influences exposure risk. Higher order carnivores had significantly higher seroprevalence than medium-sized browser species. The highest seroprevalence observed in an intermediate host was 71% in spotted-tailed quolls (Dasyurus maculatus), the largest mammalian mesopredator, in areas of low cat density. Mesopredator release of cats may be a significant issue for native species conservation, potentially affecting the population viability of many endangered species.
1. Introduction
Wildlife disease is a key threatening process for species conservation. Evidence suggests that diseases are increasing in the face of escalating environmental degradation and worldwide homogenisation of ecosystems (Daszak, 2000). Increased exposure to feral and domestic animals (e.g. Thorne and Williams, 1988, Dobson and Foufopoulos, 2001) and biodiversity loss (Keesing et al., 2010) have both been associated with increased disease risks. Additionally, they have played a primary role in many recent emerging diseases that have resulted in significant population declines (Schrag and Wiener, 1995), including canine distemper in lions (Panthera leo) (Kissui and Packer, 2004) and black footed ferrets (Mustela nigripes) (Thorne and Williams, 1988). There is a critical need to understand the context in which diseases operate in changing ecosystems and the potential threats posed to susceptible populations (Schrag and Wiener, 1995, Woodroffe, 1999, Keesing et al., 2010).
Changes in ecosystems can increase transmission and prevalence of diseases by either changing host or vector ecology, including abundance and behaviour, or by compromising immune function through stress (Schrag and Wiener, 1995, Dobson and Foufopoulos, 2001, Keesing et al., 2010). Hence, biodiversity loss that can reduce competitive pressure on reservoir hosts and thereby increase their population density, has the potential to increase pathogen transmission (Keesing et al., 2010), amplifying the abundance of infectious agents in the environment (Scott, 1988). Feral animals in particular are an important factor in disease emergence. These animals can facilitate both the transmission of pathogens to naive hosts through contact with both domestic species and wildlife as well as the introduction of new pathogens to naive wild animal populations (Dobson and Foufopoulos, 2001, Kissui and Packer, 2004).
The obligate coccidian parasite Toxoplasma gondii is particularly important as an example of a pathogen of conservation significance spread by feral animals. Members of the cat family (Felidae) are the only known definitive hosts, but nearly all warm-blooded animals can act as intermediate hosts. T. gondii infection is widespread and has been proposed as a conservation threat arising from feral cats, as indicated by clinical and subclinical infections observed in wild intermediate host-species ranging from Australian marsupials (e.g. Attwood et al., 1975, Johnson et al., 1989, Obendorf et al., 1996), to dolphins (e.g. Inskeep et al., 1990), sea otters (Enhydra lutris nereis) (Cole et al., 2000, Miller et al., 2002), new world monkeys (e.g. Dietz et al., 1997) and many avian species (Dubey, 2002). Most mammals, including humans, which are infected with T. gondii have asymptomatic infections; however, the virulence of the strain of the parasite and the susceptibility of the host can affect pathogenicity within a host (Innes, 1997, Hill et al., 2005, Parameswaran et al., 2010). T. gondii is of particular concern for immune-compromised individuals, pregnant females (Dubey, 1991, Innes, 1997, Hill et al., 2005) and wildlife species which have not co-evolved with felids and their parasites, such as Australian marsupials (Johnson et al., 1988, Innes, 1997). The transmission route may be through the ingestion of oocysts, the infective and environmentally resilient stage of the T. gondii parasite, excreted in the millions by a single cat and remaining viable in vegetation, soil and water for up to a year in favourable conditions (Dubey, 1991, Hill et al., 2005). Alternatively, infection can occur through ingestion of tissue cysts in infected meat (Hill et al., 2005) or by vertical transmission from mother to offspring (Parameswaran et al., 2009b).
In addition to overt increases in mortality or morbidity, pathogens can affect hosts in subtle ways including secondary costs associated with greater energy investment in immune responses, changes in anti-predator behaviours or reduced breeding success and competitive fitness (Scott, 1988, Lafferty et al., 2006), placing species at greater risk of extinction from other threatening processes (Woodroffe, 1999, Lafferty and Gerber, 2002). The cost of latent T. gondii infection is poorly known in intermediate hosts, with studies on rats indicating behavioural manipulation by the parasite to increase transmission, including decreased anti-predator behaviour, reduced inhibition towards novel objects and greater activity levels (Webster, 1994, Webster, 2001, Berdoy et al., 2000). Subclinical infections have also been linked to an increased incidence of brain cancer (Thomas et al., 2012), slower reaction times (Havlicek et al., 2001) and mental illness in humans (Torrey and Yolken, 2003).
T. gondii is of concern for Australian native marsupials, which appear to be particularly susceptible to acute infection (Johnson et al., 1988, Innes, 1997, Parameswaran et al., 2010, Thompson et al., 2010). Due to Australia’s geographic isolation, native species remained unexposed to T. gondii until the domestic cat (Felis catus) was introduced into Australia at the time of European settlement about 200 years ago (Abbott, 2002). There are many reports of deaths in Australian marsupials attributed to toxoplasmosis (e.g. Canfield et al., 1990, Hartley et al., 1990, Obendorf and Munday, 1990), and anecdotal evidence links T. gondii with range contractions and population extinctions in some native species (Shepherd and Mahood, 1978, Braithwaite and Griffiths, 1994, Obendorf et al., 1996, Thompson et al., 2010). Eastern barred bandicoots (Perameles gunni) have been shown to succumb to experimental T. gondii infection within a few days, without producing IgG antibodies (Bettiol et al., 2000), and evidence demonstrates that this species may not have the ability to maintain a long-term subclinical T. gondii infection (Obendorf et al., 1996). Seroprevalence of T. gondii in several native Australian marsupials varies greatly among populations. This may relate to the local distribution of feral cat populations and conditions affecting the persistence of oocysts in the environment (Arundel et al., 1977, Parameswaran, 2008). The individual and population level impacts of both acute and latent T. gondii infection on wild Australian native species or on any naive host species worldwide are poorly understood.
In Tasmania, the small island state of Australia, a unique opportunity exists to study disease ecology associated with changes in definitive host populations. Populations of feral cats are increasing (Hollings et al., submitted for publication) following a severe disease-induced population decline of the apex mammalian predator, the Tasmanian devil (Sarcophilus harrisii), from devil facial tumour disease (DFTD), an unusual transmissible cancer (Hawkins et al., 2006, Lachish et al., 2007, McCallum et al., 2007). This increase in feral cat populations appears to be an instance of mesopredator release, a phenomenon in which smaller predators increase in abundance following the decline of an apex predator. Mesopredator release (sensu Soule et al., 1988) has been reported from a range of global ecosystems with wide ranging effects including species extinctions (Crooks and Soule, 1999, Johnson et al., 2007) and changes in community composition (Courchamp et al., 1999, Terborgh et al., 2001, Beschta and Ripple, 2009).
To the best of our knowledge, the direct effects of apex predator loss and mesopredator release on changing disease dynamics have not been examined. The aim of this study is to assess whether the density of feral cats is linked to the seroprevalence of T. gondii infection in Tasmanian wildlife, to determine whether an increasing feral cat population may correspond to an increased level of risk to naive native marsupials of contracting acute toxoplasmosis. We also assess the seroprevalence in different trophic levels and then assess seroprevalence in a model species in relation to feral cat densities from two different sample sources: road kill and animals culled under permit.
2. Materials and methods
2.1. Blood samples
To determine links between cat density and infection with T. gondii, blood samples were collected from larger marsupial herbivores across Tasmania: common brushtail possums (Trichosurus vulpecula, ∼3 kg; n = 14); Tasmanian pademelons (Thylogale billardierii, males = ∼8 kg, females = ∼4 kg; n = 228); and Bennetts wallabies (Macropus rufogriseus males = ∼15 kg, females = ∼11 kg; n = 25). Herbivores were considered to be the most suitable species for this part of the study as exposure is through contact with oocysts in the environment, including vegetation and water, without the confounding effect of the consumption of infected prey, although possums are partially omnivorous. Smaller native mammals were not assessed due to difficulties in testing for latent infection in these species. Blood samples were collected both opportunistically from fresh road-kill and more systematically from animals culled by commercial and private shooters holding permits for crop and forestry grazing protection. Road-kill samples were taken from the heart within several hours of death, ensuring that blood was still fluid. Where possible in culling programs, samples were taken from multiple individuals of the same species from each localised area, which were at least 5 km apart from each other. Samples from these animals were collected from free flowing blood from wounds or from the heart immediately after death. Following initial results, efforts were focused on collecting samples from Tasmanian pademelons. This species has a previously reported T. gondii antibody prevalence of 18% using an enzyme-linked immunosorbent assay (ELISA) test (Johnson et al., 1988). Samples for pademelons were taken over the entire northern half of Tasmania within a two year period.
In addition, we collected blood samples from other marsupial species to establish seroprevalence of T. gondii at different trophic levels. Samples were tested from three higher-order marsupial carnivores from several locations in Tasmania: the Tasmanian devil (males = ∼8 kg, females = ∼ 6 kg; n = 18); the spotted-tail quoll (Dasyurus maculatus; males = ∼4 kg, females = ∼2 kg; n = 7); and the eastern quoll (Dasyurus viverrinus; males = ∼1 kg, females = ∼0.8 kg; n = 24). All samples for these species were obtained by ear vein puncture during live trapping. Tasmanian devil and spotted-tailed quolls samples were collected in relatively low cat density areas mostly in the north-west (Fig. 1, Fig. 2). Eastern quolls were sampled in areas of relatively lower and higher cat density in the north-west and south of the state (Fig. 1, Fig. 2). Additional samples (n = 17) were collected from an offshore island, Bruny Island, where Tasmanian devils have never been present. T. gondii seroprevalence in these samples was compared to that in eastern quolls from Tasmanian mainland sites but was not included in the overall trophic analysis.
Fig. 1.
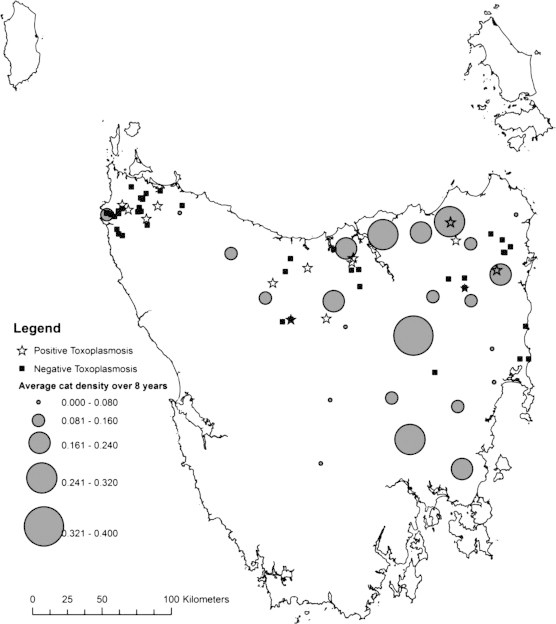
Map of Tasmania showing average cat densities from individual spotlighting districts over 8 years and blood collection sites for the Tasmanian pademelon. Positive T. gondii sites are those where at least one sample tested positive to IgG antibodies. Negative sites are those where no evidence of exposure to T. gondii was found in any sample.
Fig. 2.
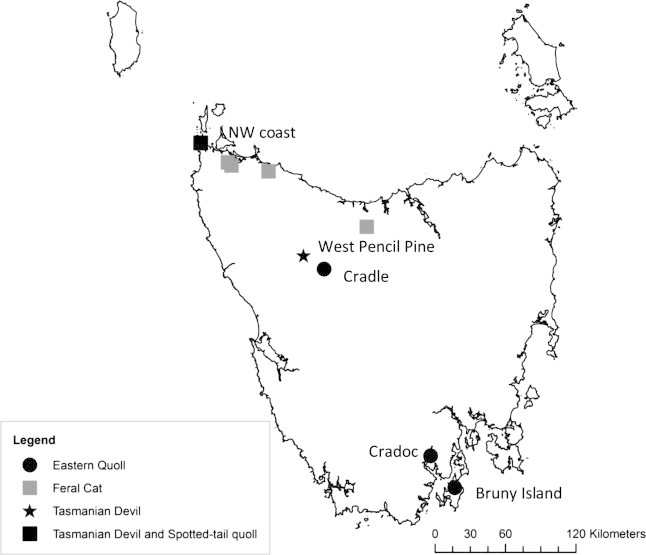
Map of Tasmania showing blood collection sites for the three native carnivore species and the introduced feral cat. Places identified are those referred to in the text.
Samples from feral cats, the definitive host of T. gondii, were taken opportunistically by commercial shooters. All of the samples were obtained in the north-west region which remains DFTD free and exhibits low cat density (Fig. 1).
2.2. Testing for T. gondii
We tested blood samples for IgG antibodies, the class of antibodies representing the secondary immune response, using a modified agglutination test (MAT) (BioMerieux, France). The addition of beta-mercaptoethanol in the MAT test destroys non-specific IgM antibodies, reducing the risk of false-positive results (Desmonts and Remington, 1980). MAT testing has been used extensively for detection of exposure to T. gondii in marsupials (Obendorf et al., 1996, Hartley and English, 2005, Eymann et al., 2006, Parameswaran et al., 2009a), as it does not require a species specific reagent and is highly sensitive. The MAT test has high specificity and there is no evidence for cross-reactivity with related organisms (Dubey et al., 1995, Dubey, 1997). Samples were tested at three different sera dilutions; 1:16, 1:64, 1:256. A positive reaction was acknowledged if agglutination occurred at a sera dilution of at least 1:64 (Obendorf et al., 1996, Hartley, 2006). A subsample of blood sera (5%) was retested with the MAT test for verification at the Animal Health Laboratory of the Department of Primary Industries, Parks, Water and Environment (DPIPWE), Tasmania.
2.3. Approximating cat density
We used a long-term state-wide spotlighting dataset to estimate feral cat density across Tasmania. The night-time spotlighting survey is conducted annually by DPIPWE, and covers more than 170 transects, each 10 km long. Individual spotlighting transects are grouped into 29 defined districts based on their proximity to each other, with each district having between 3 and 8 separate transects (Hocking and Driessen, 1992). All non-domestic species are recorded in addition to environmental variables that may affect animal detectability or activity level, including moonlight, wind speed and rainfall (Hocking and Driessen, 1992).
The total number of cats observed over an eight year period on all transects within each spotlight district was divided by the total number of transects surveyed to determine an index of cat density for each district (Fig. 1). The eight year period was chosen to allow for the life expectancy of all the species surveyed for T. gondii infection. This timeframe was considered appropriate as animals may be exposed even before they leave the pouch through vertical transmission (Parameswaran et al., 2009b), and IgG antibodies can remain present for life (Remington et al., 2004). For some districts DFTD was present for varying lengths of time during this 8 year period. The aggregation of data also reduced some of the issues with yearly variation, such as moonlight and rainfall, from surveying transects only once per year.
For a more detailed analysis of seroprevalence variation in response to cat density, all spotlighting transects that fell within a 10 km radius of each blood collection site were identified. An estimate of local cat density was calculated by dividing the number of cats detected on each transect within the 10 km zone during the eight year period by the total number of transects surveyed in the zone. Additionally, environmental variables which were hypothesised to affect the persistence of oocysts in the environment and positively influence cat density were included in the models. These are: the percentage of open vegetation (comprising largely of agricultural land), the average annual rainfall, and the number of address points (a proxy for human settlement and urbanisation), and they were determined for a 2 km buffer around the sites.
2.4. Statistical analysis
2.4.1. Cat density and T. gondii infection
For a broad scale analysis pademelon samples were divided by location into three geographical regions: north-east, central and north-west. These regions were selected to represent different lengths of time DFTD has been present which corresponds to the length of time and magnitude of Tasmanian devil population decline and potentially of mesopredator release of cats. DFTD has been present in the north-east region since (∼1996–1999), with declines of up to 94% in some areas (Hawkins et al., 2006, McCallum et al., 2007). The disease arrived in the central region between approximately 2000 and 2007 and has also caused substantial population declines (>50%), whereas the north-west region currently remains free of the disease and maintains a large population of Tasmanian devils. Fisher’s exact test (in R version 2.11.0) was used to assess evidence of significant differences in T. gondii seroprevalence between geographic regions. This analysis was done using all of the pademelon samples and then repeated using only the culled samples to remove the potentially confounding collection method. An index of cat density was obtained for each of the three geographic regions by adding up the total number of cats sighted within the region and dividing by the total number of transects surveyed within the eight year period.
Seroprevalences in culled and road-kill pademelons were compared using Fisher’s exact test. For a finer scale analysis, we used generalised linear models (GLMs) with a binomial error structure and complementary log log link to model the seroprevalence of T. gondii in pademelons as a function of cat density, the source of the sample (road-kill or culled) and environmental variables, specifically the geographic region, the percentage of open vegetation, the average annual rainfall and the number of address points.
There were 65 individual samples from a total of 228 which either had no spotlighting transects that fell within the 10 km radius or where we had only approximate co-ordinates; these samples were excluded from the analysis. We used weights (wi) derived from small sample corrected Akaike Information Criterion (AICc) to evaluate support for alternative models, where the weight indicates the relative support for each model (Burnham and Anderson, 2002). The relative importance of each explanatory variable was quantified by summing the weights of all models containing the variable (Burnham and Anderson, 2002, Rhodes et al., 2006).
2.4.2. Trophic level analysis
Fisher’s exact test was also used to compare T. gondii seroprevalence among different hosts within a trophic level, i.e. within carnivores and browsers, and then between different trophic levels.
In addition, a generalised linear mixed model (GLMM) with a binomial error structure and complementary log log link function (using the ‘nlme’ library in R version 2.11.0) was used to assess whether T. gondii seroprevalence in the carnivore guild, comprising the Tasmanian devil, spotted-tailed quoll and eastern quoll, was significantly different from the herbivore guild, comprising the brushtail possum, Bennett’s wallaby and Tasmanian pademelon. Seroprevalence was the binary response variable where presence or absence of T. gondii infection in the individual was noted as 1 or 0 respectively. In this analysis, the guild was the fixed effect and the species within the guilds was the random effect.
3. Results
3.1. Seroprevalence of T. gondii
Toxoplasma seroprevalences are reported in Fig. 3. Very low T. gondii seroprevalence in brushtail possums (0/14 samples analysed) and Bennetts wallabies (2/25 samples analysed) found in preliminary results would render detection of state-wide regional differences in seroprevalence in these species difficult even with large sample sizes. As a result, we focused subsequent blood sampling on pademelons.
Fig. 3.
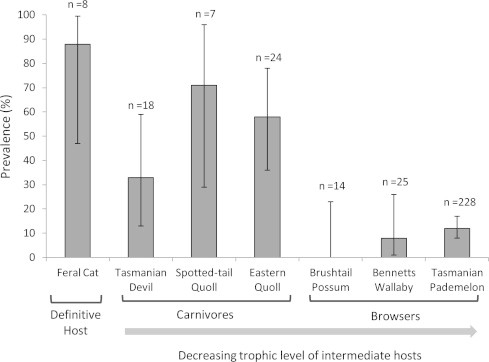
Prevalence of IgG antibodies of Tasmanian mammals to T. gondii by trophic level; n represents the total number of samples tested. Standard error bars are shown.
There were significant differences between the three geographic regions in T. gondii seroprevalence (p = 0.035), with the north-west having the lowest seroprevalence (7%), followed by the north-east (15%) and the highest seroprevalence was recorded in the central region (19%) (Table 1). This regional difference was even stronger when assessing only culled pademelons (p = 0.020; north-west 6%, central 20%, north-east 22%). Cat density over the last eight years was the lowest in the north-west region, and approximately half of that in the central and north-east regions (Fig. 1 and Table 1).
Table 1.
Prevalence of IgG antibodies to T. gondii in Tasmanian pademelons. Cat density is the estimated value of the number of cats per transect within the region for data aggregated over 8 years.
| Region | Number positive (n) | Total number (n) | Percent positive (%) | Est. cat density (cats per transect) |
|---|---|---|---|---|
| North-east | 6 | 39 | 15 | 0.13 |
| Central | 14 | 74 | 19 | 0.13 |
| North-west | 8 | 115 | 7 | 0.07 |
| Total | 28 | 228 | 12 | |
The seroprevalence of T. gondii in road-kill samples from pademelons was considerably higher (31%, n = 16) than culled animals (11%, n = 212). The Fisher’s exact test indicated significantly different seroprevalence between the two sample sources (p = 0.033). However, the low number of road-kill samples relative to the culling samples means that this result should be interpreted with some caution.
From the GLM analysis using the 10 km zones, there were nine models with a ΔAICc of less than two from the best supported model. Cat density, sample source, geographic region and annual rainfall were all contained within these top models, indicating some influence of these predictor variables on T. gondii seroprevalence in pademelons (Table 2). The source of the samples and the DFTD region were the two most influential predictor variables with a relative importance of 81% and 64% respectively. Cat density had a relative importance of 47% and rainfall 52%.
Table 2.
The most parsimonious GLM models of factors influencing the seroprevalance of T. gondii in pademelons. k = number of model parameters, ΔAICc represents the difference in AICc from the most parsimonious model, wi = model weight.
| Model parameters | k | ΔAICc | wi |
|---|---|---|---|
| Sample source + region | 3 | 0.00 | 0.12 |
| Cat density + rainfall + sample source | 4 | 0.04 | 0.12 |
| Cat density + rainfall + sample source + region | 5 | 0.61 | 0.09 |
| Cat density + sample source + region | 4 | 0.89 | 0.08 |
| Rainfall + sample source + region | 4 | 1.07 | 0.07 |
| Region | 2 | 1.17 | 0.07 |
| Method | 2 | 1.52 | 0.06 |
| Rainfall + method | 3 | 1.53 | 0.06 |
| Cat density + method | 3 | 1.70 | 0.05 |
| Null | 1 | 3.77 | 0.02 |
3.2. Trophic level differences
Different levels of the food web had significantly different seroprevalence levels of T. gondii (Fig. 3). Higher order mammalian carnivores, the Tasmanian devil (33%) , spotted-tail quoll (71%) and the smaller meso-carnivore, the eastern quoll (58%) had a significantly greater likelihood of being seropositive (Fig. 3) than the omnivorous brushtail possum (0%) and both wallabies, the Tasmanian pademelon (12%) and the larger Bennett’s wallaby (8%), (GLMM model, herbivore est. = −1.50 ± 0.27, p < 0.0001; Fishers 2 sided, p < 0.0001). There were no significant differences amongst species within the higher order carnivore trophic level (p = 0.16, Fisher’s 2 sided), or amongst the three species classified as browsers (p = 0.44, Fisher’s 2 sided). The spotted-tail quoll had the highest seroprevalence level of any species except cats, but sample size was relatively small (n = 7). High seroprevalences were also recorded in eastern quolls, with 72% at Cradoc (n = 11) and 46% at Cradle (n = 13). On Bruny Island however, seroprevalence in this species was only 12% (n = 17). The largest or top order mammalian predator, the Tasmanian devil, had the lowest seroprevalence of all carnivores overall. The seroprevalence in this host however indicated high levels of site variability between the two sites where Tasmanian devils were surveyed with 60% at West Pencil Pine (n = 5) and 23% at the north-west coast (n = 13). Similar evidence for large differences in site seroprevalences was observed in eastern quolls. All samples of the two highest order intermediate host species, the Tasmanian devil and spotted-tailed quoll, were taken from the north-west region, where cat density was relatively low. For the definitive host, the feral cat, seven of the eight samples were seropositive for T. gondii. The majority of these samples were obtained in the north-west region which has lowest feral cat density (Fig. 1).
4. Discussion
Seroprevalence of T. gondii in Tasmanian pademelons and cat population density are both higher in the north-east and central regions of Tasmania than in the north-west region. This suggests that the population density of the definitive host might play an important role in the transmission of T. gondii. Any increase in feral cat populations through mesopredator release, therefore, has the potential to increase the risk of T. gondii exposure in some naive native intermediate host species. Mammals at higher trophic levels exhibited significantly higher seroprevalence. However, neither high prevalence levels nor pathogenicity of parasites are a good gauge of the effect of disease on a population (McCallum and Dobson, 1995), and the population level impact of T. gondii infection on naive native host species, or indeed any host species remains unknown.
Feral cats in Tasmania appear to have consistently high seroprevalence of T. gondii, and despite the small sample sizes from our study, the results support similar evidence from an earlier study which found T. gondii antibodies in 51 of 53 Tasmanian feral cats using the indirect fluorescent-antibody test (Gregory and Munday, 1976). This suggests that feral cats can have a high risk of infection even where they occur at low densities. Consequently, lower seroprevalence levels in intermediate hosts in areas with lower cat densities compared with areas of higher cat densities may result from reduced contamination of the environment with oocysts, rather than a minimised risk of infection for cats, for whom carnivorism is likely an important route for acquiring T. gondii. Vertical transmission may also play a role in macropods, maintaining high prevalence even in the absence of a definitive host (Parameswaran et al., 2009b), but the implications of this mode of transmission for the persistence of T. gondii infections in wildlife populations remains unknown. Further, the high genetic diversity of T. gondii in Australian wildlife leaves open the possibility that felids may not be the only definitive host for the genus in Australia (Pan et al., 2012). Because of the limited data available for estimating cat density, we were not able to assess in detail the magnitude of the effect of higher cat densities on T. gondii seroprevalence in Tasmanian marsupials and further investigation of this possible link is justified. The spotlighting dataset also has inherent issues with internal variability associated with different observers and weather conditions among other factors (Driessen and Hocking, 1992).
The seroprevalence of T. gondii in road-kill animals was significantly higher than in culled animals. This suggests that pademelons with T. gondii infection may be more at risk of being killed on the road than uninfected individuals. Unfortunately, the sample size of road-killed animals was small, but this intriguing result warrants further investigation. Latent infections in rodents and humans have revealed evidence of behavioural changes such as slower reaction times. Within the prey size range of feral cats, rats with subclinical T. gondii infections are at greater risk of predation (Webster, 1994, Berdoy et al., 2000). Even in humans otherwise asymptomatic infected individuals have decreased reaction times (Havlicek et al., 2001) and have an increased risk of car accidents (Flegr et al., 2002). Although disease has been proposed as a cause of sudden population declines of some Australian marsupials (e.g. Abbott, 2006) subclinical infections are rarely considered. T. gondii may be an example of a pathogen that even in the absence of clinical effects can cause behavioural changes that decrease survival, and individuals with subclinical infections could be more susceptible to predation.
Among Tasmanian marsupials the higher order carnivores had a significantly higher seroprevalence of T. gondii infection than the herbivores, from which we infer that the risk of infection with T. gondii increases at higher levels of the food-web. Exposure to infected wild prey or carrion explains high prevalence in carnivores (Dubey, 1991, Dubey et al., 1999). Carnivores at the top of the food-web may receive continuous and concentrated doses of infectious agents in their diet (Munsen, 2003), likely explaining the increasing vulnerability to parasites with increasing trophic level (Lafferty et al., 2006). Infection in carnivores and scavengers is particularly common with extremely high prevalence reported and infection levels are a good indicator of prevalence of T. gondii in the environment (Dubey et al., 1999, Hill et al., 2005). The high seroprevalence of T. gondii in carnivores likely reflects its presence in prey and also in the environment. The MAT test, which destroys IgM antibodies, measures exposure to a parasite, rather than a recent infection; this means seroprevalence essentially reflects a lifetime of exposure because IgG antibodies can persist for life (Remington et al., 2004). The marsupial carnivores we surveyed were mostly in the north-west where cat densities are generally lower (Fig. 1) and seroprevalence may be higher in other areas of Tasmania where cat densities are higher. The effects of T. gondii infections at both an individual and population level, and whether seroprevalence levels provide an indication of potential risks to these species are unknown.
In coyotes and red foxes in North America, prevalence in the larger more dominant predator (coyote 59%) was lower than in the smaller mesopredator (red fox 86%) (Dubey et al., 1999). In our sample, T. gondii prevalence was higher in the smaller native mesopredators than the apex predator, the Tasmanian devil, but this was not statistically significant. With carnivores, the number of prey items may be more important than the density of the definitive host in exposure to T. gondii because the primary route of infection may be through eating infected prey, rather than through environmental contamination with oocysts. Smaller, more numerous and varied prey species typical of mesopredators may in part explain the higher seroprevalence for these species. Pathogen transmission is not always linked to host density (Keesing et al., 2010) and prevalence of generalist pathogens may remain high even if the density of any particular host species is low (Packer et al., 2003).
Different host species may exhibit variability in susceptibility to infection by a parasite in terms of resistance (variability in propensity to become infected) and in tolerance (variability in harm caused by a given level of infection) (Raberg et al., 2009). For T. gondii and naive marsupials, there is evidence of variability in both tolerance and resistance. Jurd (1994) reported that the immune response of marsupials is both slower and less accentuated than in eutherian species. Indeed, Bettiol et al. (2000) found that eastern barred bandicoots had died from primary T. gondii infection before detectable antibodies had been produced. T. gondii itself may increase susceptibility in some marsupial hosts, following natural selection of strains that are highly transmissible between genetically similar hosts (Parameswaran et al., 2010). When assessing prevalence in Australian marsupials, macropod species may not be equally susceptible to infection, with smaller host species more vulnerable than larger counterparts to acute toxoplasmosis (Dubey and Crutchley, 2008). These differences in susceptibility were also found in the present study. The results are consistent with previous studies, which found prevalence in wild brushtail possums in a major urban area of just over 6% (Eymann et al., 2006) and prevalence in Bennetts wallabies from Tasmania of 3% (Johnson et al., 1988). In pademelons T. gondii seroprevalence of 18% has been reported (Johnson et al., 1988) and they have shown evidence of clinical signs in the wild (Obendorf and Munday, 1983, Johnson et al., 1989). Such differences may be a consequence of smaller species grazing closer to the soil increasing their risk of ingesting oocysts or through selection of parasite lineages which have increased transmissibility among hosts with a specific genetic makeup (Parameswaran et al., 2010).
Disease studies using prevalence indices may risk bias from both abiotic and biotic factors which result in differences in detection probabilities (Jennelle et al., 2007). Serological and MAT tests for T. gondii in wild animals may underestimate seroprevalence (Owen and Trees, 1998). The under estimation of seroprevalence may result from individuals infected through vertical transmission that can exhibit a tolerance to the infection (Owen and Trees, 1998, Parameswaran et al., 2009b). Age may also be a confounding factor in assessments of seroprevalence as the likelihood of coming into contact with T. gondii would increase over the lifetime of a host. Age is unlikely to be a significant factor in this study as methodology was standardised across all areas. Future research on T. gondii-induced mortality in marsupial population declines need to consider variations in susceptibility to the parasite, host ecology and environmental stressors.
For diseases that have density dependent transmission dynamics, higher host densities will enhance transmission rates and lead to increased likelihood of elevated parasite loads per individual host and disease outbreaks (Scott, 1988, Dobson and Foufopoulos, 2001). Increased cat abundance can be managed by implementing cat control programs, but caution must be exercised before these are initiated for any species to reduce disease transmission. Culling may have complex effects on host behaviour, for example by increasing animal movements and thus facilitating disease transmission (Donnelly et al., 2003). Currently the implications of management and control practices on the behaviour of feral cats are unknown and should be further investigated.
Species that flourish with environmental degradation and habitat destruction may be those responsible for magnifying transmission of pathogens (Keesing et al., 2010). Feral cats are a common species not only in Australia but worldwide in areas of human habitation and are resilient to environmental changes. They have been shown to respond positively to the loss of larger native predators in a variety of ecological systems (e.g. Crooks and Soule, 1999, Hollings et al., submitted for publication, Kennedy et al., 2012). Ecosystem changes, including loss of the apex predator, could provide an advantage to some parasite species, including T. gondii, if they facilitate transmission (Dobson and Foufopoulos, 2001, Horwitz and Wilcox, 2005). Recent epidemics which have significantly reduced populations have highlighted the need for better understanding of wildlife disease ecology. Parasites are an integral part of ecosystems and communities, affecting ecosystem heath and regulation. They are susceptible to the same environmental processes as other larger species which dominate conservation programmes. As the environment is continually altered through anthropogenic processes it is likely that infectious diseases will continue to emerge, and the potential of these pathogens to significantly affect the population viability of many endangered species, already suffering from overwhelming environmental pressures will escalate (Cleaveland et al., 2001, Dobson and Foufopoulos, 2001).
Acknowledgements
We would first like to acknowledge the Holsworth Wildlife Research Endowment for funding this research. Analysis of the data was supported by ARC Discovery Grant DP110103069. We would like to thank Shannon Troy, Rodrigo Hamede and Bronwyn Fancourt for collecting carnivore samples, and especially Forestry Tasmania and private shooters who allowed us to collect samples from culled macropods. We would like to acknowledge the DPIPWE Animal Health Laboratory with special mention to Pat Statham and Sarah Peck for all manner of help and advice in sample collection and analysis. Finally, we would like to acknowledge DPIPWE for providing the spotlighting data necessary in the analysis.
References
- Abbott I. Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildlife Research. 2002;29:51–74. [Google Scholar]
- Abbott I. Mammalian faunal collapse in Western Australia, 1875–1925: the hypothesised role of epizootic disease and a conceptual model of its origin, introduction, transmission, and spread. Australian Zoologist. 2006;33:530–561. [Google Scholar]
- Arundel J.H., Barker I.K., Beveridge I. In: The Biology of Marsupials. Stonehouse B., Gilmore D., editors. University Park Press; Baltimore: 1977. Diseases of marsupials; pp. 141–154. [Google Scholar]
- Attwood H.D., Woolley P.A., Rickard M.D. Toxoplasmosis in dasyurid marsupials. Journal of Wildlife Diseases. 1975;11:543–551. doi: 10.7589/0090-3558-11.4.543. [DOI] [PubMed] [Google Scholar]
- Berdoy M., Webster J.P., McDonald D.W. Fatal attraction in rats infected with Toxoplasma gondii: a case of parasite manipulation of its mammalian host. Proceedings of the Royal Society B: Biological Sciences. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschta R.L., Ripple W.J. Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biological Conservation. 2009;142:2401–2414. [Google Scholar]
- Bettiol S.S., Obendorf D.L., Nowarkowski M., Goldsmid J.M. Pathology of experimental toxoplasmosis in Eastern barred Bandicoots in Tasmania. Journal of Wildlife Diseases. 2000;36:141–144. doi: 10.7589/0090-3558-36.1.141. [DOI] [PubMed] [Google Scholar]
- Braithwaite R.W., Griffiths A.D. Demographic variation and range contraction in the northern quoll, Dasyurus hallucatus (Marsupialia: Dasyuridae) Wildlife Research. 1994;21:203–217. [Google Scholar]
- Burnham K.P., Anderson D.R. second ed. Springer-Verlag; New York: 2002. Model Selection and Multimodal Inference: A Practical Information and Theoretic Approach. [Google Scholar]
- Canfield P.J., Hartley W.J., Dubey J.P. Lesions of toxoplasmosis in Australian marsupials. Journal of Comparative Pathology. 1990;103:159–167. doi: 10.1016/s0021-9975(08)80172-7. [DOI] [PubMed] [Google Scholar]
- Cleaveland S., Laurenson M.K., Taylor L.H. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R.A., Lindsay D.S., Howe D.K., Roderick C.L., Dubey J.P., Thomas N.J., Baeten L.A. Biological and molecular characterizations of Toxoplasma gondii strains obtained from southern sea otters (Enhydra lutris nereis) Journal of Parasitology. 2000;86:526–530. doi: 10.1645/0022-3395(2000)086[0526:BAMCOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Courchamp F., Langlais M., Sugihara G. Cats protecting birds: modelling the mesopredator release effect. Journal of Animal Ecology. 1999;68:282–292. [Google Scholar]
- Crooks K.R., Soule M.E. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–566. [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife - Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Desmonts G., Remington J.S. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. Journal of Clinical Microbiology. 1980;11:562–568. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz H.H., Henriksen P., Bille-Hansen V., Henriksen S.A. Toxoplasmosis in a colony of New World monkeys. Veterinary Parasitology. 1997;68:299–304. doi: 10.1016/s0304-4017(96)01088-6. [DOI] [PubMed] [Google Scholar]
- Dobson A., Foufopoulos J. Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2001;356:1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C.A., Woodroffe R., Cox D.R., Bourne J., Gettinby G., Le Fevre A.M., McInerney J.P., Morrison W.I. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature. 2003;426:834–837. doi: 10.1038/nature02192. [DOI] [PubMed] [Google Scholar]
- Driessen M.M., Hocking G.J. Department of Parks, Wildlife and Heritage; Tasmania: 1992. Review and Analysis of Spotlight Surveys in Tasmania: 1975–1990. [Google Scholar]
- Dubey J.P. Toxoplasmosis – an overview. The Southeast Asian Journal of Tropical Medicine and Public Health. 1991;22(Suppl.):88–92. [PubMed] [Google Scholar]
- Dubey J.P. Validation of the specificity of the modified agglutination test for toxoplasmosis in pigs. Veterinary Parasitology. 1997;71:307–310. doi: 10.1016/s0304-4017(97)00016-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. A review of toxoplasmosis in wild birds. Veterinary Parasitology. 2002;106:121–153. doi: 10.1016/s0304-4017(02)00034-1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Crutchley C. Toxoplasmosis in wallabies (Macropus rufogriseus and Macropus eugenii): blindness, treatment with atovaquone, and isolation of Toxoplasma gondii. Journal of Parasitology. 2008;94:929–933. doi: 10.1645/GE-1448.1. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Thulliez P., Weigel R.M., Andrews C.D., Lind P., Powell E.C. Sensitivity and specificity of various serologic tests for detection of Toxoplasma gondii infection in naturally infected sows. American Journal of Veterinary Research. 1995;56:1030. [PubMed] [Google Scholar]
- Dubey J.P., Storandt S.T., Kwok O.C.H., Thulliez P., Kazacos K.R. Toxoplasma gondii antibodies in naturally exposed wild coyotes, red foxes, and gray foxes and serologic diagnosis of toxoplasmosis in red doxes fed T. gondii oocysts and tissue cysts. Journal of Parasitology. 1999;85:240–243. [PubMed] [Google Scholar]
- Eymann J., Herbert C.A., Cooper D.W., Dubey J.P. Serologic survey for Toxoplasma gondii and Neospora caninum in the common brushtail possum (Trichosurus vulpecula) from urban Sydney, Australia. Journal of Parasitology. 2006;92:267–272. doi: 10.1645/GE-709R.1. [DOI] [PubMed] [Google Scholar]
- Flegr J., Havlicek J., Kodym P., Maly M., Smahel Z. Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC Infectious Diseases. 2002;2:11. doi: 10.1186/1471-2334-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory G.G., Munday B.L. Internal parasites of feral cats from the Tasmanian midlands and King Island. Australian Veterinary Journal. 1976;52:317–320. doi: 10.1111/j.1751-0813.1976.tb02396.x. [DOI] [PubMed] [Google Scholar]
- Hartley M.P. Toxoplasma gondii infection in two common wombats (Vombatus ursinus) Australian Veterinary Journal. 2006;84:107–109. doi: 10.1111/j.1751-0813.2006.tb12242.x. [DOI] [PubMed] [Google Scholar]
- Hartley M., English A. A seroprevalence survey of Toxoplasma gondii in common wombats (Vombatus ursinus) European Journal of Wildlife Research. 2005;51:65–67. [Google Scholar]
- Hartley W.J., Dubey J.P., Spielman D.S. Fatal toxoplasmosis in Koalas (Phascolarctos cinereus) The Journal of Parasitology. 1990;76:271–272. [PubMed] [Google Scholar]
- Havlicek J., Gasova Z., Smith A.P., Zvara K., Flegr J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology. 2001;122:515–520. doi: 10.1017/s0031182001007624. [DOI] [PubMed] [Google Scholar]
- Hawkins C.E., Baars C., Hesterman H., Hocking G.J., Jones M.E., Lazenby B., Mann D., Mooney N., Pemberton D., Pyecroft S., Restani M., Wiersma J. Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation. 2006;131:307–324. [Google Scholar]
- Hill D.E., Chirukandoth S., Dubey J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Animal Health Research Reviews/Conference of Research Workers in Animal Diseases. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- Hocking G.J., Driessen M.M. Department of Parks, Wildlife and Heritage; Hobart, Tasmania: 1992. Tasmanian Spotlight Survey Manual. [Google Scholar]
- Hollings, T., Jones, M., Mooney, N., McCallum, H., submitted for publication. Ecosystem impacts of disease induced apex predator decline: the Tasmanian devil and devil facial tumour disease (DFTD).
- Horwitz P., Wilcox B.A. Parasites, ecosystems and sustainability: an ecological and complex systems perspective. International Journal for Parasitology. 2005;35:725–732. doi: 10.1016/j.ijpara.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Innes E.A. Toxoplasmosis: comparative species susceptibility and host immune response. Comparative Immunology, Microbiology and Infectious Diseases. 1997;20:131–138. doi: 10.1016/s0147-9571(96)00038-0. [DOI] [PubMed] [Google Scholar]
- Inskeep W., Gardiner C.H., Harris R.K., Dubey J.P., Goldston R.T. Toxoplasmosis in Atlantic bottle-nosed dolphins (Tursiops truncatus) Journal of Wildlife Diseases. 1990;26:377–382. doi: 10.7589/0090-3558-26.3.377. [DOI] [PubMed] [Google Scholar]
- Jennelle C.S., Cooch E.G., Conroy M.J., Senar J.C. State-specific detection probabilities and disease prevalence. Ecological Applications. 2007;17:154–167. doi: 10.1890/1051-0761(2007)017[0154:sdpadp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Johnson A.M., Roberts H., Munday B.L. Prevalence of Toxoplasma gondii antibody in wild macropods. Australian Veterinary Journal. 1988;65:199–201. doi: 10.1111/j.1751-0813.1988.tb14456.x. [DOI] [PubMed] [Google Scholar]
- Johnson C.N., Isaac J.L., Fisher D.O. Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proceedings of the Royal Society B: Biological Sciences. 2007;274:341–346. doi: 10.1098/rspb.2006.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.M., Roberts H., Statham P., Munday B.L. Serodiagnosis of acute toxoplasmosis in macropods. Veterinary Parasitology. 1989;34:25–33. doi: 10.1016/0304-4017(89)90160-x. [DOI] [PubMed] [Google Scholar]
- Jurd R.D. “Not proper mammals”: immunity in monotremes and marsupials. Comparative Immunology, Microbiology and Infectious Diseases. 1994;17:41–52. doi: 10.1016/0147-9571(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Keesing F., Belden L.K., Daszak P., Dobson A., Harvell C.D., Holt R.D., Hudson P., Jolles A., Jones K.E., Mitchell C.E., Myers S.S., Bogich T., Ostfeld R.S. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M., Phillips B.L., Legge S., Murphy S.A., Faulkner R.A. Do dingoes suppress the activity of feral cats in northern Australia? Austral Ecology. 2012;37:134–139. [Google Scholar]
- Kissui B.M., Packer C. Top-down population regulation of a top predator: lions in the Ngorongoro Crater. Proceedings of the Royal Society B: Biological Sciences. 2004;271:1867–1874. doi: 10.1098/rspb.2004.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachish S., Jones M., McCallum H. The impact of disease on the survival and population growth rate of the Tasmanian devil. Journal of Animal Ecology. 2007;76:926–936. doi: 10.1111/j.1365-2656.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Gerber L.R. Good medicine for conservation biology: the intersection of epidemiology and conservation theory. Conservation Biology. 2002;16:593–604. [Google Scholar]
- Lafferty K.D., Dobson A.P., Kuris A.M. Parasites dominate food web links. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H., Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends in Ecology and Evolution. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- McCallum H., Tompkins D.M., Jones M., Lachish S., Marvanek S., Lazenby B., Hocking G., Wiersma J., Hawkins C.E. Distribution and impacts of Tasmanian devil facial tumor disease. EcoHealth. 2007;4:318–325. [Google Scholar]
- Miller M.A., Gardner I.A., Kreuder C., Paradies D.M., Worcester K.R., Jessup D.A., Dodd E., Harris M.D., Ames J.A., Packham A.E., Conrad P.A. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) International Journal for Parasitology. 2002;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Munson, L., 2003. Scope and magnitude of disease in species conservation. In: Armstrong, D., Jakob-Hoff, R., Seal, U.S., (Eds.), Animal movements and disease risk: a workbook. Conservation Breeding Specialist Group (SSC/IUCN), pp. 19–21.
- Obendorf D.L., Munday B.L. Toxoplasmosis in wild Tasmanian wallabies. Australian Veterinary Journal. 1983;60:62. doi: 10.1111/j.1751-0813.1983.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Obendorf D.L., Munday B.L. In: Bandicoots and Bilbies. Seebeck J.H., Brown P.R., Wallis R.L., Kemper C.M., editors. Surrey Beatty and Sons; Sydney: 1990. Toxoplasmosis in wild eastern barred bandicoots, Perameles gunnii; pp. 193–197. [Google Scholar]
- Obendorf D.L., Statham P., Driessen M. Detection of agglutinating antibodies to Toxoplasma gondii in sera from free-ranging eastern barred bandicoots (Perameles gunnii) Journal of Wildlife Diseases. 1996;32:623–626. doi: 10.7589/0090-3558-32.4.623. [DOI] [PubMed] [Google Scholar]
- Owen M.R., Trees A.J. Vertical transmission of Toxoplasma gondii from chronically infected house (Mus musculus) and field (Apodemus sylvaticus) mice determined by polymerase chain reaction. Parasitology. 1998;116:299–304. doi: 10.1017/s003118209700231x. [DOI] [PubMed] [Google Scholar]
- Packer C., Holt R.D., Hudson P.J., Lafferty K.D., Dobson A.P. Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecology Letters. 2003;6:797–802. [Google Scholar]
- Pan S., Thompson R.C.A., Grigg M.E., Sundar N., Smith A. Western Australian marsupials are multiply infected with genetically diverse strains of toxoplasma gondii. PLoS ONE. 2012;7:e45147. doi: 10.1371/journal.pone.0045147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N. Murdoch University; Perth: 2008. Toxoplasma gondii in Australian Marsupials. [Google Scholar]
- Parameswaran N., O’Handley R.M., Grigg M.E., Fenwick S.G., Thompson R.C.A. Seroprevalence of Toxoplasma gondii in wild kangaroos using an ELISA. Parasitology International. 2009;58:161–165. doi: 10.1016/j.parint.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., O’Handley R.M., Grigg M.E., Wayne A., Thompson R.C.A. Vertical transmission of Toxoplasma gondii in Australian marsupials. Parasitology. 2009;136:939–944. doi: 10.1017/S0031182009006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., Thompson R.C.A., Sundar N., Pan S., Johnson M., Smith N.C., Grigg M.E. Non-archetypal Type II-like and atypical strains of Toxoplasma gondii infecting marsupials of Australia. International Journal for Parasitology. 2010;40:635–640. doi: 10.1016/j.ijpara.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberg L., Graham A.L., Read A.F. Decomposing health: tolerance and resistance to parasites in animals. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J.S., Thulliez P., Montoya J.G. Recent developments for diagnosis of toxoplasmosis. Journal of Clinical Microbiology. 2004;42:941. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J.R., Wiegand T., McAlpine C.A., Callaghan J., Lunney D., Bowen M., Possingham H.P. Modeling species’ distributions to improve conservation in semiurban landscapes: koala case study. Conservation Biology. 2006;20:449–459. doi: 10.1111/j.1523-1739.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Schrag S.J., Wiener P. Emerging infectious disease: what are the relative roles of ecology and evolution? Trends in Ecology and Evolution. 1995;10:319–324. doi: 10.1016/s0169-5347(00)89118-1. [DOI] [PubMed] [Google Scholar]
- Scott M.E. The impact of infection and disease on animal populations: implications for conservation biology. Conservation Biology. 1988;2:40–56. [Google Scholar]
- Shepherd N.C., Mahood I.T. The potential effect of feral dogs and cats on Australian native fauna. Australian Advances in Veterinary Science. 1978:108. [Google Scholar]
- Soule M.E., Bolger D.T., Allison C.A., Wright J., Sorice M., Hill S. Reconstructed dynamics of rapid extinctions of chaparral-requiring birds in urban habitat islands. Conservation Biology. 1988;2:75–92. [Google Scholar]
- Terborgh J., Lopez L., Nunez P.V., Rao M., Shahabuddin G., Orihuela G., Riveros M., Ascanio R., Adler G.H., Lambert T.D., Balbas L. Ecological meltdown in predator-free forest fragments. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- Thomas F., Lafferty K.D., Brodeur J., Elguero E., Gauthier-Clerc M., Misse D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biology Letters. 2012;8:101–103. doi: 10.1098/rsbl.2011.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C.A., Lymbery A.J., Smith A. Parasites, emerging disease and wildlife conservation. International Journal for Parasitology. 2010;40:1163–1170. doi: 10.1016/j.ijpara.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Thorne E.T., Williams E.S. Disease and endangered species: the black-footed ferret as a recent example. Conservation Biology. 1988;2:66–74. [Google Scholar]
- Torrey E.F., Yolken R.H. Toxoplasma gondii and schizophrenia. Emerging Infectious Diseases. 2003;9:1375–1380. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J.P. The effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology. 1994;109:583–589. doi: 10.1017/s0031182000076460. [DOI] [PubMed] [Google Scholar]
- Webster J.P. Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microbes and Infection. 2001;3:1037–1045. doi: 10.1016/s1286-4579(01)01459-9. [DOI] [PubMed] [Google Scholar]
- Woodroffe R. Managing disease threats to wild mammals. Animal Conservation. 1999;2:185–193. [Google Scholar]


