Graphical abstract
Keywords: Red fox, Helminth parasites, Zoonotic, Environmental correlates
Highlights
-
•
We investigated the helminth fauna of red foxes from 14 locations in southwest WA.
-
•
Environmental variables influence parasite occurrence.
-
•
Host factors did not influence parasite presence.
-
•
An extension of geographic range of flukes Plagiorchis maculosus and Brachylaima cribbi.
-
•
Echinococcus granulosus and Taenia ovis were not detected.
Abstract
Red foxes (Vulpes vulpes) are the most common and widely distributed wild carnivore worldwide. These predators harbour a wide range of parasites, many of which may have important conservation, agricultural and zoonotic repercussions. This project investigated the occurrence of helminth parasites from the intestines of 147 red foxes across 14 sampling localities of southwest Western Australia. Helminth parasites were detected in 58% of fox intestines: Dipylidium caninum (27.7% of foxes), Uncinaria stenocephala (18.2%), Toxocara canis (14.9%), Spirometra erinaceieuropaei (5.4%), Toxascaris leonina (4.7%), Taenia serialis (1.4%), Taenia hydatigena (0.7%), unidentified Taenia spp. (4.1%), Brachylaima cribbi (0.7%), Plagiorchis maculosus (0.7%) and an Acanthocephalan; family Centrorhynchidae (2.1%). Importantly, two cestodes of agricultural significance, Echinococcus granulosus and Taenia ovis, were not detected in red foxes in this study, despite the presence of suitable intermediate hosts in the diets of these animals. Parasite richness varied from 1–3 species per host, with average parasite number varying from 1–39 worms (across all helminth species). Regression analyses indicated that the presence of four helminth parasites was related to various environmental factors. The presence of S. erinaceieuropaei (p < 0.001), T. leonina (p < 0.01) and U. stenocephala (p < 0.01) was positively associated with average relative humidity which may affect the longevity of infective stages in the environment. The presence of S. erinaceieuropaei and U. stenocephala (p < 0.001) was positively associated with 5-y-average minimum temperature which could reflect poor survival of infective stages through cold winter conditions. The presence of T. canis and U. stenocephala (p < 0.001) was positively associated with the percentage cover of native vegetation at each sampling location, which is likely to reflect transmission from native prey species acting as paratenic hosts. These data identify environmental factors affecting transmission and potential distribution of each parasite taxon, and provide important information increasing our understanding of the potential effects of environmental change on parasite ecology.
1. Introduction
The red fox (Vulpes vulpes) is widely distributed and abundant on every major continent throughout the world except Antarctica (Strahan, 1983; Long, 1988; Dickman, 1996). This generalist predator competes with and predates upon a wide range of native and livestock species as well as playing an important role in disease transmission. Red foxes are known to harbour parasites of agricultural, conservation and zoonotic importance (Wolfe et al., 2001; Henderson, 2009). Understanding the factors that influence the persistence and spread of these parasites is important in maintaining wildlife community health and may have implications for transmission to livestock and humans.
Many parasite species require relatively specific environmental conditions to complete their lifecycles. Therefore, adverse conditions can affect the number of viable parasites in the environment as well as the rate of development of infective stages. Climatic variables such as rainfall, temperature, humidity and barometric pressure can therefore influence the geographic distribution of parasites as much as host presence (Stromberg, 1997). Climate change is of increasing importance to determining the occurrence and impacts of infectious diseases. With the expected increase in frequency and severity of extreme climatic events as a result of climate change, geographic distributions of both parasites and their hosts are also expected to change (Morgan and Wall, 2009; Polley and Thompson, 2009). These changing distributions may lead to parasites switching to newly available and/or naïve host species with deleterious effects (Polley and Thompson, 2009). Host characteristics (e.g. age and sex) may also influence an animal’s susceptibility to infection (Mourand and Poulin, 1998; Behnke et al., 1999; Chowdhury et al., 2001).
Despite the widespread distribution and importance of the red fox as a pest species in Australia, few studies have investigated the helminth parasites they harbour. In particular, little is known regarding the climatic variables that influence the presence and transmission of parasite taxa within Australian environments. Given the propensity for a changing climate to influence these environments, information regarding factors that influence parasite occurrence is required. The aim of this study was to investigate the gastrointestinal helminths present in red foxes in southwest Western Australia and the factors influencing parasite presence. We predicted an effect of environmental conditions (temperature, rainfall and humidity) on the presence of parasite species which have an environmental stage, and predicted an effect of the percentage cover of native vegetation on the presence of parasite species that rely on native species as intermediate hosts.
2. Materials and methods
2.1. Sample collection
Red fox carcasses (n = 124) were sourced from a coordinated culling program across 14 locations within the intensive land use zone of southwest Western Australia (over two designated weekends in February and March 2010), with additional samples (n = 23) obtained via opportunistic collection i.e. road kill or private culling operations during the same months (Table 1). Necropsies were conducted on red fox carcasses within 12 h of being shot. The animal’s sex was recorded and body mass (kg), head length (cm), head-body length (cm) and pes length (cm) were measured. The entire gastrointestinal (GI) tract (including stomach, duodenum, ileum, caecum and colon) was removed and stored according to means available until examination: on ice for ∼8 h, transferred to a 4 °C fridge, or transferred immediately to a −18 °C freezer taken into the field. Given the variability in stomach content, the dissected GI tract mass was subtracted from overall body mass for statistical analyses.
Table 1.
Environmental and climatic measures for each sampling location and number of samples collected.
| Location | % Native vegetation covera | Avg. humidity for previous 6 mo (%)b | Temperature |
5-y-avg. Annual rainfall (mm) | Avg. rainfall for previous 6 m (mm) | ||
|---|---|---|---|---|---|---|---|
| 5-y-avg. Mean (°C) | 5-y-avg. Min. (°C) | 5-y-avg. Max. (°C) | |||||
| Armadale (n = 2) | 30.91 | 62.67 | – | 11.1 | 24.3 | 765.88 | 17.13 |
| Boyup brook (n = 11) | 37.21 | 70.83 | 23.08 | 8.72 | 22.9 | 587.52 | 10.88 |
| Corrigin (n = 18) | 5.91 | 56.17 | 24.3 | 9.96 | 24.3 | 355.5 | 12.67 |
| Darkan (n = 30) | 25.52 | 56.17 | 23.08 | 9.74 | 23.08 | 534.86 | 19.47 |
| Dumbleyung (n = 16) | 7.83 | 56.17 | 23.08 | 9.74 | 23.08 | 337.84 | 9.21 |
| Frankland (n = 1) | 32.05 | 66.5 | 20.5 | 9.5 | 20.5 | 597.2 | 10.17 |
| Gingin (n = 13) | 46.36 | 47.67 | 25.58 | 10.62 | 25.28 | 577.76 | 19.17 |
| Katanning (n = 14) | 10.86 | 58.33 | 22.58 | 9.14 | 22.18 | 454.44 | 17.77 |
| Kemerton (n = 1) | 33.77 | 56.5 | – | 10.78 | 22.84 | 768.54 | 15.05 |
| Mt. Barker (n = 12) | 30.04 | 69.67 | 20.64 | 9.72 | 20.64 | 634.66 | 27.35 |
| Nyabing (n = 3) | 9.74 | 58.33 | 22.18 | 9.14 | 22.18 | 360.58 | 12.13 |
| Quairading (n = 13) | 4.79 | 56 | 25.82 | 10.02 | 25.9 | 340.06 | 15.87 |
| Williams (n = 5) | 18.54 | 53.17 | – | 9.68 | 22.84 | 452.46 | 14.9 |
| Woodanilling (n = 6) | 10.49 | 58.33 | 22.58 | 9.14 | 22.18 | 417.52 | 14.52 |
Percentage native vegetation cover was calculated within a 30 km radius of each sampling location (data sourced from Shepherd et al., 2001).
Climatic measures were sourced from Bureau of Meteorology (Department of Sustainability, 2010).
Skulls were collected for estimation of age (Forbes-Harper, 2010). In Australia most fox cubs are born in August and September (Saunders et al., 1995), and therefore tend to form a tight age cohort. Ages are indicated to the nearest year: at the time we sampled, foxes in their first year were aged between 5 and 10 months old (mo), those in their second year were 17–22 mo, etc. Animals <2 years old (yo) could generally be aged via cranial sutures: foxes with an open basisphenoid–basioccipital suture were considered in their first year and those where this suture was closed but the presphenoid–basisphenoid suture remained open were considered in their second year (Harris, 1978). Animals >2 yo were aged by counting the numbers of dentine layers of the canine teeth following the method of Roulichova and Andera (2007). Age, sex and body mass details for the animals sampled are shown in Table 2.
Table 2.
Age, weight and sex breakdown of samples collected (n = 147).
| Range | No. of foxes | |
|---|---|---|
| Age cohort | <2 years | 105 |
| 2–4 years | 26 | |
| 4–6 years | 4 | |
| 6–8 years | 3 | |
| Unknown | 9 | |
| Weight | <5 kg | 47 |
| 5–8 kg | 96 | |
| >8 kg | 2 | |
| Sex | Male | 80 |
| Female | 67 | |
2.2. Examination of gastrointestinal tracts
Frozen GI tracts were defrosted at room temperature (20–30 °C) overnight. Stomach contents were examined for a separate diet analysis; parasites present in the stomach were separated from stomach contents and identified as part of the present study. Intestines were laid out on a tray, the small and large intestines opened longitudinally and then the entire GI tract was cut into ∼25 cm-long sections. Each section was methodically examined under a dissecting microscope (magnification varying from 1× to 4×). Additionally, intestinal walls were scraped with soft forceps to remove mucous and food items and to enable the detection of parasites attached to the intestinal mucosa. Parasites imbedded within food particles were gently extracted. Parasites were counted and transferred to 70% ethanol or 10% neutral buffered formalin (cestodes only). Only scoleces and heads were counted and used for parasite intensity. Some parasites that were found were unable to be unidentified due to poor/degraded specimens. Specimens found in only one individual with only a single worm can be put down to artefact from diet.
Parasites were identified based on morphological characteristics. Nematodes and acanthocephalans were cleared in lactophenol prior to identification. Identification of nematodes was made based on characteristics outlined in Bowman and Georgi (2009) and Schmidt and Roberts (1985). Hookworms were identified by presence of a cutting plate and ascarids were identified by their tapered tail in males or egg morphology in females. Tapeworms were identified using morphological characteristics pertaining to their proglottids, as defined in Cheng (1986). The juvenile acanthocephalans were identified to family according to the key in Yamaguti (1963) and Amin (1987). The keys used in the basic identification for trematodes were from Schell (1970). The trematodes were stained with Semichon’s acetocarmine (Plagiorchis maculosus) and Harris’s haematoxylin (Brachylaima cribbi) respectively. Both of these flukes were identified from the position of the uterus and ventral sucker and measurements for identification were compared to Krasnolobova (1977) and Butcher and Grove (2001), respectively. Individual Taenia were identified to species based on morphological characters, i.e. anterior rostellar hooks from hook squashes (Beveridge and Gregory, 1976); however there is some overlap in hook lengths between species of Taenia and some specimens collected were not whole.
2.3. Statistical analysis
Backwards-stepwise logistic regression analyses (Statistica Version 9; StatSoft Inc., 2001) were performed to determine factors correlated with the presence/absence (1/0) of the five most common parasite species (i.e. species that had a prevalence of >4%: Dipylidium caninum, Spirometra erinaceieuropaei, Toxocara canis, Toxascaris leonina, and Uncinaria stenocephala) as the dependent variables. Twelve independent variables tested for each dependent variable included seven environmental and climatic measures recorded for each sampling location (Table 1), and five intrinsic factors (body mass, head-body length, pes length, sex, and age). The analyses were performed on the presence/absence in individual host animals (not prevalence by location), to avoid biassing results due to differences in sample size between locations. Each individual animal was attributed the environmental values for the site of capture (we could not include location as a random factor in these analyses since each location had unique environmental values and would therefore have confounded the results of the analyses).
Correlation between the presence of each parasite species (only parasites that were present in at least three foxes) was examined using a Pearson’s correlation matrix (Excel 2007; Microsoft), based on the presence/absence data for each individual fox examined. A Bonferroni correction was applied.
Mixed-model ANOVA (Statistica Version 9; StatSoft Inc., 2001) was performed to examine whether the load of each of the five most common parasite species had a detrimental effect upon host body condition (i.e. body mass). Body mass of each individual fox was used as the dependent variable. To take into account allometric relationships, three measures of body size were included in the analysis (head length, head-body length, and pes length); the inclusion of multiple body size indices improves how allometric change is accounted for (Green, 2001). The age (year; fixed continuous covariate) and sex (fixed categorical variable) of the host were also included in the analyses; location was included as a random factor to account for repeated samples from each location. The load of five parasites (D. caninum, S. erinaceieuropaei, T. canis, T. leonina, U. stenocephala) were included as fixed continuous covariates (only parasites species that were present in at least three foxes were included in the analysis).
3. Results
3.1. Helminth species presence, prevalence and infracommunity richness
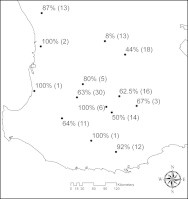
Fifty-eight percent of foxes (n = 85 of 147 total) harboured helminth parasites within their GI tract (Fig. 1). Fourteen helminth parasite taxa were recovered from red foxes across all sampling locations; five cestodes, five nematodes, three trematodes and one acanthocephalan (Table 3).
Fig. 1.
Prevalence of helminths in red foxes (n=147) from sampling locations throughout southwest Western Australia. Numbers in parentheses indicate sample size at each location.
Table 3.
Prevalence and parasite intensity of helminth species in red foxes. Species within each Family are sorted in decreasing order of prevalence.
| Phylum | Parasite | Prevalence (%) | Helminth parasite intensity |
||||
|---|---|---|---|---|---|---|---|
| Average | SD | Min | Max | Median | |||
| Nematoda | Uncinaria stenocephala | 18.2 | 17.00 | 17.33 | 1 | 78 | 12 |
| Toxocara canis | 14.9 | 7.00 | 7.84 | 1 | 34 | 4 | |
| Toxascaris leonina | 4.7 | 1.00 | – | 1 | 1 | 1 | |
| Unknown roundworm | 1.4 | 1.00 | – | 1 | 1 | 1 | |
| Unknown hookworm | 0.7 | 1.00 | – | 1 | 1 | – | |
| Cestoda | Dipylidium caninum | 27.7 | 8.00 | 12.14 | 1 | 51 | 2 |
| Spirometra erinaceieuropei | 5.4 | 3.00 | 1.98 | 1 | 6 | 3 | |
| Taenia serialis | 1.4 | 39.00 | 49.50 | 4 | 74 | 39 | |
| Taenia hydatigena | 0.7 | 1.00 | – | 1 | 1 | – | |
| Taenia spp. | 4.1 | 1.00 | – | 1 | 1 | 1 | |
| Trematoda | Brachylaima cribbi | 0.7 | 6.00 | – | 6 | 6 | – |
| Plagiorchis maculosus | 0.7 | 8.00 | – | 8 | 8 | – | |
| Unknown fluke | 0.7 | 1.00 | – | 1 | 1 | – | |
| Acanthocephala | Centrorhynchidaea | 2.1 | 2.00 | 1.73 | 1 | 4 | 1 |
Identified to family only.
Of the helminth species recovered, D. caninum was the most prevalent (present in 27.7% of 147 red foxes examined) and widespread (9 of 14 locations), followed by U. stenocephala (18.2%; 7 locations) and T. canis (14.9%; 5 locations). The three trematode species (B. cribbi, P. maculosus and a uterus of an unidentified fluke) were each found from only a single host. The Acanthocephalan (2.0%; 3 locations), could only be identified to family (Centrorhynchidae) due to them being juvenile specimens. Of the 86 foxes that harboured parasites, single parasite species infections (53 individuals) were more common than mixed. Mixed infections consisted of either two species (n = 25) or a maximum of three parasite species (n = 7).
3.2. Worm burden
The parasite intensity (burden) of helminths from all foxes was highly variable (Table 3). The parasite species with the highest maximum intensity was U. stenocephala, with 78 worms detected within a single fox. The maximum helminth intensity for T. serialis, D. caninum and T. canis was 74, 51 and 34, respectively. Eleven parasite species were identified with a minimum parasite intensity of one worm in a host. The mean intensity of each individual parasite species varied from 1 to 39 individuals.
3.3. Factors associated with the presence of parasites
Backward stepwise multiple regression analyses identified a significant association between environmental measures and the presence of four of the five most prevalent parasite species (Table 4; the presence of D. caninum was not attributable to any of the factors tested). The sites where T. leonina was present typically had higher average relative humidity than those sites where T. leonina was not detected (p < 0.01). The sites where S. erinaceieuropaei was present were more humid (p < 0.001) with warmer minimum temperatures (p < 0.001). The sites where U. stenocephala was present were more humid (p < 0.01), had warmer minimum temperatures (p < 0.001), and had more native vegetation present (p < 0.001) than other sites. Those sites that had a greater percentage prevalence of T. canis generally had more native vegetation present (p < 0.001).
Table 4.
Summary of five separate backwards stepwise multiple logistic regression analyses carried out to determine factors that were associated with the presence/absence of the five most prevalent parasite species. This table shows beta coefficient values ± standard errors.
| Parasite species | D. caninum | S. erinaceieuropei | T. canis | T. leonina | U. stenocephala |
|---|---|---|---|---|---|
| Environmental factors | |||||
| % Native vegetation cover (30km radius) | – | – | 0.455 ± 0.079⁎⁎⁎ | – | 0.493 ± 0.073⁎⁎⁎ |
| Avg. monthly rainfall- previous 6 mo (mm) | – | – | – | – | – |
| 5-y-avg. annual rainfall (mm) | – | – | – | – | – |
| Avg. humidity- previous 6 mo (%) | – | 0.574 ± 0.115⁎⁎⁎ | – | 0.285 ± 0.085⁎⁎ | 0.343 ± 0.10⁎⁎ |
| 5-y-avg. mean temp. (°C) | – | – | – | – | – |
| 5-y-avg. min temp. (°C) | – | 0.494 ± 0.115⁎⁎⁎ | – | – | 0.364 ± 0.103⁎⁎⁎ |
| 5-y-avg. max temp. (°C) | – | – | – | – | – |
| Host factors | |||||
| Sex | – | – | – | – | – |
| Head/body length (cm) | – | – | – | – | – |
| Pes length (cm) | – | – | – | – | – |
| Body mass (minus GI tract mass, kg) | – | – | – | – | – |
| Age (years) | – | – | – | – | – |
Factors that were eliminated as part of the backwards stepwise regression are indicated with –; significant factors are indicated with asterisks.
p < 0.01.
p < 0.001.
3.4. Parasite correlations
A correlation matrix analysis identified correlations between parasite occurrences in foxes (Table 5). A significant correlation was observed with the occurrence of S. erinaceieuropaei and U. stenocephala within foxes/across sampling locations (p < 0.001). A strong positive correlation was also observed between S. erinaceieuropaei and T. leonina; T. canis and U. stenocephala; T. leonina and U. stenocephala (p < 0.01). A strong negative correlation was observed between D. caninum and U. stenocephala (p < 0.01).
Table 5.
Pearson’s correlation matrix with a Bonferroni correction between parasites in red foxes (n = 147) based on presence/absence data. Only parasites present in at least 3 foxes were included in the analysis.
| Dipylidium caninum | Taenia spp. | Spirometra erinaceieuropaei | Toxocara canis | Toxascaris leonina | Uncinaria stenocephala | |
|---|---|---|---|---|---|---|
| Dipylidium caninum | 1 | |||||
| Taenia spp. | −0.052 | 1 | ||||
| Spirometra erinaceieuropaei | −0.149 | 0.102 | 1 | |||
| Toxocara canis | −0.091 | −0.087 | 0.067 | 1 | ||
| Toxascaris leonina | −0.139 | −0.046 | 0.228⁎⁎ | −0.004 | 1 | |
| Uncinaria stenocephala | −0.217⁎⁎ | −0.098 | 0.351⁎⁎⁎ | 0.244⁎⁎ | 0.224⁎⁎ | 1 |
Statistically significant correlations are indicated with asterisks.
p < 0.01.
p < 0.001.
3.5. Body condition
Body condition of foxes was not associated with the load of each of five parasite species (Mixed-model ANOVA D. caninum p = 0.283, S. erinaceieuropaei p = 0.137, T. canis p = 0.282, T. leonina p = 0.537, U. stenocephala p = 0.383), once allometric relationships (head length p < 0.001, head-body length p < 0.001, pes length p = 0.360) and age of the foxes (p = 0.019) were taken into account.
4. Discussion
The overall prevalence in this study is considerably lower than that of studies from eastern Australia: Ryan (1976) found an 80.6% prevalence of helminths in foxes (n = 180) and Coman (1973) detected a prevalence of 71% (n = 1320). Helminth prevalence in red foxes from other countries are also high; (100% for Spain: Gortázar et al., 1998; 96% for Ireland; Wolfe et al., 2001; 63% for Belgium: Vervaeke et al., 2005; 98% for Denmark: Saeed et al., 2006; 84.5% for Italy: Di Cerbo et al., 2008b). These previous studies all used similar methods of studying the intestinal tract; however specific gastrointestinal methodology did vary; i.e. intestinal scrapings and examination under a microscope was used in some studies or intestinal washes, where only macro parasites were recorded, in the other half. Various factors can explain the lower prevalence found in this study compared to these previous studies. Some factors include intermediate and paratenic host availability, diversity and density, host immunity and nutritional status, previous exposure to parasites, and seasonal and/or yearly fluctuations in parasite presence. Other factors include individual host factors, such as age and body size and environmental measures which have been explored in this study.
Red foxes are known to harbour a wide diversity of parasites. Despite the lower overall prevalence of helminth parasites detected in this study, red foxes harboured 14 parasitic species representing four parasitic phyla: Trematoda, Nematoda, Cestoda and Acanthocephala. The common helminth species detected in the present study (i.e. T. canis, T. leonina, U. stenocephala, D. caninum and Taenia spp.), have also been identified in previous studies (Gortázar et al., 1998; Wolfe et al., 2001; Vervaeke et al., 2005; Saeed et al., 2006), signifying that red foxes from the southwest Western Australia harbour vulpine helminths commonly found in red foxes elsewhere in the world.
Two species of trematode flukes (P. maculosus and B. cribbi) found in this study have not been previously reported from Western Australia (Angel, 1959; Butcher and Grove, 2001). P. maculosus has previously been noted as occurring in South Australia in insectivores as definitive hosts (i.e. insectivorous birds such as Willie wagtails, magpies and sparrows and some mammals), but not from red foxes in Australia (Angel, 1959). However, other Plagiorchis spp. has been found in wild canids, artic foxes and red foxes in previous studies in other countries (Rausch et al., 1983; Kapel and Nansen, 1996; Di Cerbo et al., 2008a,b). B. cribbi generally occurs in chickens, mice and rats as definitive hosts, and has also been noted from South Australia (Butcher and Grove, 2001). Both of these trematodes are known to be infective to humans. The finding of these two trematodes in foxes from southwest Western Australia expands their known geographical distribution and host range.
Even though the Acanthocephalan found was only in juvenile form, it was able to be identified to family: Centrorhynchidae. This Acanthocephalan family has been described in red foxes as well as other fox species in other parts of the world, including Europe (Eira et al., 2006) and South America (Ruas et al., 2008). The present study has reported an extension of the geographical distribution of this parasite taxon.
Parasite presence varied markedly between the 14 sampling locations. There were no detectable links with host intrinsic factors (i.e. sex, body mass, age) but various environmental factors were associated with presence of the most common parasite species. Environmental factors can strongly influence the larval and/or free living stages of parasites which may be susceptible to suboptimal temperature or humidity conditions (Stromberg, 1997). In addition to these environmental factors influencing parasite larval stages, host availability and behaviour can also be affected by environmental factors and therefore influence the occurrence and persistence of parasites (Stromberg, 1997; Gortázar et al., 1998; Hegglin et al., 2007).
High humidity levels positively influence parasite survival during their larval and free living stages as it invariably relates to higher moisture levels in micro-environments, particularly in soils, leading to an increased survival rate as well as dispersion of infective stages (Onorato, 1932; Stromberg, 1997). In dry environments, eggs and larvae are more susceptible to desiccation and eggs are unlikely to embryonate (Ruiz de Ybanez et al., 2001). In the present study, average relative humidity for the previous 6 mo at each location had a significant influence on three parasite species (S. erinaceieuropaei; T. leonina; and U. stenocephala) within red foxes. This correlation has previously been noted for U. stenocephala (Stromberg, 1997). Although their presence in this study was linked with higher humidity, T. leonina eggs are able to tolerate greater climatic variation than eggs from parasites such as T. canis, thus increasing their potential distribution and transmission risk (Sprent and Barrett, 1964; Okulewicz et al., 2012). Humidity may also correlate with the presence of habitat suitable for paratenic and intermediate hosts thereby increasing parasite numbers (Ryan, 1976). For example, S. erinaceieuropaei has a complicated life cycle utilising numerous paratenic hosts, including waterborne copepods and arthropods. The increased survival and transmission of this parasite in three locations (Gin Gin, Mount Barker and Perth metropolitan area) in the southwest is indicative of suitable habitat and conditions for parasite development and transmission.
In addition to humidity, extremes of temperature (high or low) are also important as they can lead to desiccation of eggs and larval stages or arrested development of infective stages in the environment (Onorato, 1932). Extremes of temperature can also determine the presence and density of suitable paratenic hosts and therefore the parasite community present. Higher minimum temperature (averaged over last 5 years) at sampling locations was significantly correlated with U. stenocephala and S. erinaceieuropaei presence in red foxes in the present study. Whilst lower minimum temperatures may be more conducive to reduced levels of desiccation (exposure of eggs and larvae to high levels of solar radiation increases susceptibility to desiccation), it can also potentially depress embryonation (Onorato, 1932) as well as larval activity and motility (Stromberg, 1997). Therefore higher average minimum temperatures are presumably favourable to the development and transmission of parasite species which involve a free-living or environmental stage.
Vegetation provides vital habitat diversity resulting in micro environments and climates within a landscape as well as refugia for potential host species (Dubinský et al., 1995). Two helminth parasites (U. stenocephala and T. canis) were positively correlated with a higher native vegetation cover. Life cycle stages for T. canis and U. stenocephala are both susceptible to desiccation and therefore vegetation cover may increase the potential for their persistence within the landscape. Dubná et al. (2007) demonstrated a higher incidence and prevalence of T. canis in parks or areas with a high vegetation cover compared to rural areas. Given the extent of clearing of native vegetation in the wheatbelt region (>90%) for agricultural purposes (DEP, 1997), it is not surprising that remnant vegetation has an important influence on the presence of parasite species (whether it is due to an increased concentration of potential intermediate hosts or more amenable environmental conditions) (Stromberg, 1997; Mizgajska, 2001).
Generally, worm intensities in the definitive host are dependent on the mode of transmission of a particular parasite (Ryan, 1976), and a high worm burden within a fox is likely to reflect the intensity of infection of the intermediate host. An example of this is D. caninum which was abundant in foxes, most likely due to a high intensity of D. caninum in the intermediate flea hosts (observed to cause flea bite dermatitis in numerous individuals) or a high infestation of fleas on the fox (Coman, 1973; Nichol et al., 1981). Similarly a high burden of S. erinaceieuropaei may also represent an individual with an opportunistic feeding preference as this parasite has a wide range of paratenic hosts including frogs, mice and lizards. The potential for infection is increased by non-specific transmission of the larval S. erinaceieuropaei to a multitude of hosts, and may be further increased by predation of these hosts by foxes. A definitive host with a high parasite burden can also infer a host that has an impaired immune system (Chandra, 1981).
The impact of parasite intensity on red fox body condition has previously been shown to have no effect (Vervaeke et al., 2005). This finding has been further confirmed in the present study with no significant correlation between the load of common parasites and fox condition (once body size and age were taken into account). Foxes in this study were generally in good condition however, it is suggested that larger body sizes and better body conditions convey a greater number and variety of niches in which the parasite can reside (Mourand and Poulin, 1998). It also agrees with the idea that as these parasites are using foxes as a definitive host, they have a minimal effect on the host so that the cycling of this parasite can continue. This result may also reflect the overriding effect of environmental rather than host factors. It may also indicate that there are other factors that may affect parasite presence that need to be explored for example host immunity status and intermediate host availability.
Significant correlations between multiple parasite species were found for red foxes. A negative association was observed between D. caninum and U. stenocephala indicating these two parasites showed minimal geographic overlap (Fig. 2). U. stenocephala was present in coastal locations and sites typified by higher vegetation cover while D. caninum was present in locations further inland (part of the intensive wheatbelt area), which had lower percentage vegetation cover. The infective life cycle stage of U. stenocephala is a free living larvae which requires specific environmental conditions in order to survive whilst D. caninum eggs, deposited in faeces, require ingestion by a flea larva which subsequently infects the definitive host. Other factors could influence the distribution of both these parasites species including the occurrence of fleas and other potential intermediate/paratenic hosts and the foraging behaviour of a definitive host can influence the amount of exposure to infective stages. In areas where D. caninum is present, the few patches of vegetation that are present, act as a vital refuge for definitive hosts. This leads to a higher concentration of fleas, flea eggs and intermediate parasite stages and a higher likelihood of contact of the host with the parasite. Blagburn and Dryden (2009) found that in areas where average relative humidity is less than 50%, flea eggs are more likely to desiccate and this can therefore lead to the lower transmission of D. caninum to red foxes in those areas. Only one study location was recorded to have an average relative humidity of less than 50% and nine locations harboured D. caninum.
Fig. 2.
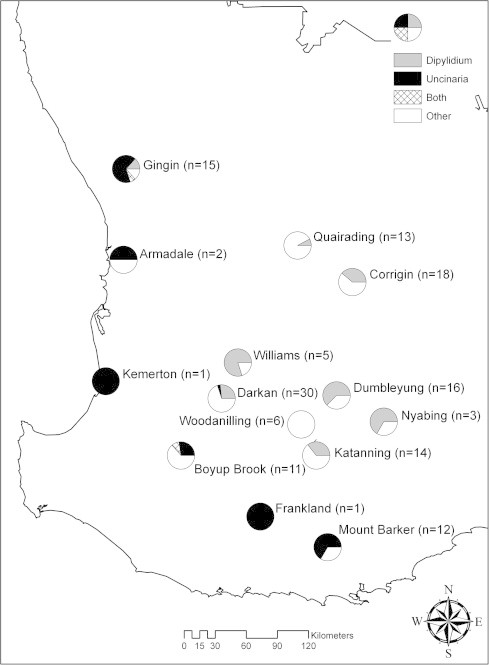
Prevalence of Uncinaria stenocephala and Dipylidium caninum from red foxes at each sampling location.
Toxocara canis has been shown to commonly occur in association with hookworms (U. stenocephala and Ancylostoma spp.) in canids, especially foxes (Newsome and Coman, 1989). Similarly, in the present study significant correlations were observed in the presence of S. erinaceieuropaei, U. stenocephala and T. leonina. These relationships may reflect the similar requirements each of these parasites have for their persistence and survival in the environment. All three parasites were significantly associated with average relative humidity and S. erinaceieuropaei and U. stenocephala were associated with warmer 5-year average minimum temperatures. This is most likely related to the sensitivity of the eggs and larval stages of these parasites to desiccation and temperature fluctuations.
Of the parasites recorded in red foxes in this study, S. erinaceieuropaei, T. canis are known to be transmissible to wildlife and livestock species, and D. caninum, S. erinaceieuropaei, T. canis, B. cribbi and P. maculosus have zoonotic potential. Canids, including red foxes, play an important role in the dissemination of a number of cestode tapeworms. In Australia, the most important of these are Echinococcus granulosus and Taenia ovis. E. granulosus persists in southwest Western Australia and is presumed to occur at a low prevalence, whilst recent statistics indicate that approximately 6% of the sheep presented at abattoirs throughout this region are currently infected with T. ovis (Palmer et al., 2013). Neither of these two parasites (body lengths: E. granulosus ∼5mm; T. ovis >5 mm) were detected in red foxes in this study, despite smaller species (P. maculosus ∼1 mm) being detected. A total of 85 Taenia worms were found in nine foxes: based on rostellar hook length 78 were identified as T. serialis, one was identified as T. hydatigena, and one was T. hydatigena/pisiformis (due to overlap in hook length) (Beveridge and Gregory, 1976). The remaining five worms were unidentifiable due to the absence of measurable scoleces. Additionally 69% of the 147 foxes examined had consumed sheep, whether consumed as carrion or freshly predated (Crawford et al., 2010). Despite the presence of T. ovis in sheep in southwest Western Australia and the high frequency of sheep in the red fox diet, neither of these parasites was present. This finding suggests that red foxes do not play an important role in the cycling of T. ovis or E. granulosus in south west WA at present. As such, domestic and/or feral dogs may play a more important role in the transmission and persistence of these two parasites in the environment.
This is the first study to have investigated the influence of environmental and host factors on the presence of helminth parasites in red foxes from southwest Western Australia. Environmental conditions were shown to have a significant effect on the presence of some species of parasites, which suggest as climatic factors shift so too could the parasite presence and prevalence. This climate change could overtly affect the transmission of larval stages and in turn lead to changing transmission strategies and eventually parasite distributions. Current control programs throughout southwest Western Australia aimed at reducing red fox impacts on environmental and agricultural resources are also assisting to help minimise disease risks. Due to the wide geographic distribution of the red fox, both in Australia and worldwide, their importance in the transmission of parasitic diseases in a changing climate should not be underestimated.
Acknowledgements
Aileen Elliot and Russell Hobbs for help in identifying helminths. Heather Crawford and Jesse Forbes-Harper for access to unpublished data. Red Card for the Red Fox control program including the farmers and volunteers who came out with us for fieldwork.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Amin O.M. Key to the families and subfamilies of Acanthocephala, with the erection of a new class (Polyacanthocephala) and a new order (Polyacanthorhynchida) J. Parasitol. 1987;73:1216–1219. [PubMed] [Google Scholar]
- Angel M. An account of Plagiorchis maculosus (Rud.), its synonymy and its life history in South Australia. Trans. R. Soc. S. Aust. 1959;82:265–281. [Google Scholar]
- Behnke J.M., Lewis J.W., Zain S.N.M., Gilbert F.S. Helminth infections in Apodemus sylvaticus in southern England: interactive effects of host age, sex and year on the prevalence and abundance of infections. J. Helminthol. 1999;73:31–44. [PubMed] [Google Scholar]
- Beveridge I., Gregory G.G. The identification of Taenia species from Australian carnivores. Aus. Vet. J. 1976;52:369–373. doi: 10.1111/j.1751-0813.1976.tb09491.x. [DOI] [PubMed] [Google Scholar]
- Blagburn B.L., Dryden M.W. Biology, treatment, and control of flea and tick infestations. Vet. Clin. N. Am. Small Anim. Pract. 2009;14:1173. doi: 10.1016/j.cvsm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Bowman D.D., Georgi J.R. Saunders; 2009. Georgis’ Parasitology for Veterinarians. [Google Scholar]
- Butcher A.R., Grove D.I. Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea:Brachylaimidae) derived from eggs recovered from human faeces in Australia. Syst. Parasitol. 2001;49:211–221. doi: 10.1023/a:1010616920412. [DOI] [PubMed] [Google Scholar]
- Chandra R. Immunocompetence as a functional index of nutritional status. Br. Med. Bull. 1981;37:89–94. doi: 10.1093/oxfordjournals.bmb.a071682. [DOI] [PubMed] [Google Scholar]
- Cheng T.C. Academic Press College Division; 1986. General Parasitology. [Google Scholar]
- Chowdhury N., O’Grady R.T.O., Sood M.L. Evolution, parasitism and host specificity in helminths. In: Chowdhury N., Tada I., editors. Perspectives on Helminthology. Science Publishers, Inc.; New Hampshire: 2001. [Google Scholar]
- Coman B.J. Helminth parasites of the fox (Vulpes vulpes) in Victoria. Aus. Vet. J. 1973;49:378–384. doi: 10.1111/j.1751-0813.1973.tb09345.x. [DOI] [PubMed] [Google Scholar]
- Crawford H., Calver M., Adams P.J., Fleming P.A. School of Biological Sciences, Murdoch University; 2010. The diet of red foxes (Vulpes vulpes) and feral cats (Felis catus) in the south west Western Australia. [Google Scholar]
- DEP . Department of Environmental Protection; Perth, WA: 1997. State of the Environment Reference Group draft working papers. Section 4, Land. State of the Environment Reporting Unit. [Google Scholar]
- Di Cerbo A.R., Manfredi M.T., Bregoli M., Milone N.F., Cova M. Wild carnivores as source of zoonotic helminths in north-eastern Italy. Helminthologia. 2008;45:13–19. [Google Scholar]
- Di Cerbo A.R., Manfredi M.T., Trevisiol K., Bregoli M., Ferrari N., Pirinesi F., Bazzoli S. Intestinal helminth communities of the red fox (Vulpes vulpes L.) in the Italian Alps. Acta Parasitol. 2008;53:302–311. [Google Scholar]
- Dickman C.R. Impact of exotic generalist predators on the native fauna of Australia. Wildl. Biol. 1996;3:165–175. [Google Scholar]
- Dubinský P., Havasiová-Reiterová K., Peťko B., Hovorka I., Tomašovičová O. Role of small mammals in the epidemiology of toxocariasis. Parasitology. 1995;110:187–193. doi: 10.1017/s0031182000063952. [DOI] [PubMed] [Google Scholar]
- Dubná S., Langrová I., Jankovská I., Vadlejch J., Pekár S., Nápravník J., Fechtner J. Contamination of soil with Toxocara eggs in urban (Prague) and rural areas in the Czech Republic. Vet. Parasitol. 2007;144:81–86. doi: 10.1016/j.vetpar.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Eira C., Vingada J., Torres J., Miquel J. The helminth community of the red fox, Vulpes vulpes, in Dunas de Mira (Portugal) and its effect on host condition. Wildl. Biol. Pract. 2006;2:26–36. [Google Scholar]
- Forbes-Harper J. School of Biology and Biotechnology Sciences, Murdoch University; Perth, WA: 2010. Ecomorphology of Red Fox (Vulpes vulpes) Skulls from South-West Western Australia (Honours thesis) [Google Scholar]
- Gortázar C., Villafuerte R., Lucientes J., Fernández-de-Luco D. Habitat related differences in helminth parasites of red foxes in the Ebro valley. Vet. Parasitol. 1998;80:75–81. doi: 10.1016/s0304-4017(98)00192-7. [DOI] [PubMed] [Google Scholar]
- Green A.J. Mass/length residuals: measures of body condition or generators of spurious results. Ecology. 2001;82:1473–1483. [Google Scholar]
- Harris S. Age determination in the Red fox (Vulpes vulpes) – an evaluation of technique efficiency as applied to a sample of suburban foxes. J. Zool. 1978;184:91–117. [Google Scholar]
- Hegglin D., Bontadina F., Contesse P., Gloor S., Deplazes P. Plasticity of predation behaviour as a putative driving force for parasite life-cycle dynamics: the case of urban foxes and Echinococcus multilocularis tapeworm. Funct. Ecol. 2007;21:552–560. [Google Scholar]
- Henderson, W., 2009. Pathogens in vertebrate pests in Australia, Invasive Animals Cooperative Research Centre, Canberra.
- Kapel C.M.O., Nansen P. Gastrointestinal helminths of Arctic foxes (Alopex lagopus) from different bioclimatological regions in Greenland. J. Parasitol. 1996;82:17–24. [PubMed] [Google Scholar]
- Krasnolobova T. Principles of the systematics of trematodes from the genus Plagiorchis Lühe, 1899. Trudy Gel’mintologicheskoî Lab. 1977;27:65–110. [Google Scholar]
- Long J.L. Agriculture Protection Board; Forrestfield, WA: 1988. Introduced Birds and Mammals in Western Australia. [Google Scholar]
- Mizgajska H. Eggs of Toxocara spp. in the environment and their public health implications. J. Helminthol. 2001;75:147–152. [PubMed] [Google Scholar]
- Morgan E.R., Wall R. Climate change and parasitic disease: farmer mitigation? Trends Parasitol. 2009;25:308–313. doi: 10.1016/j.pt.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Mourand S., Poulin R. Density, body mass and parasite richness of terrestrial mammals. Evol. Ecol. 1998;12:717–727. [Google Scholar]
- Newsome A.E., Coman B.J. Canidae. In: Walton D.W., Richardson B.J., editors. Fauna of Australia. Australian Government Publishing Service; Canberra: 1989. pp. 993–1005. (Mammalia). [Google Scholar]
- Nichol S., Ball S.J., Snow K.R. Prevalence of intestinal parasites in feral cats in some urban areas of england. Vet. Parasitol. 1981;9:107–110. doi: 10.1016/0304-4017(81)90028-5. [DOI] [PubMed] [Google Scholar]
- Okulewicz A., Perec-Matysiak A., Buńkowska K., Hildebrand J. Toxocara canis, Toxocara cati and Toxascaris leonina in wild and domestic carnivores. Helminthologia. 2012;49:3–10. [Google Scholar]
- Onorato A.R. The effects of temperature and humidity on the ova of Toxocara canis and Trichuris vulpis. Am. J. Epidemiol. 1932;16:266–287. [Google Scholar]
- Palmer D., Quai C., Butler R. Department of Agriculture and Food Western Australia; 2013. Sheep measles in Western Australia: Foxes are Unlikely to Play a Role, Ovine Observer. [Google Scholar]
- Polley L., Thompson R.C.A. Parasite zoonoses and climate change: molecular tools for tracking shifting boundaries. Trends Parasitol. 2009;25:285–291. doi: 10.1016/j.pt.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Rausch R.L., Fay F.H., Williamson F.S.L. Helminths of the arctic fox, Alopex lagopus (L.), in Greenland. Can. J. Zool. 1983;61:1847–1851. [Google Scholar]
- Roulichova J., Andera M. Simple method of age determination in red fox, Vulpes vulpes. Folia Zoolog. 2007;56:440–444. [Google Scholar]
- Ruas J.L., Muller G., Farias N.A., Gallina T., Lucas A.S., Pappen F.G., Sinkoc A.L., Brum J.G. Helminths of Pampas fox Pseudalopex gymnocercus (Fischer, 1814) and of Crab-eating fox Cerdocyon thous (Linnaeus, 1766) in the Southern of the State of Rio Grande do Sul, Brazil. Rev. Brasil. Parasitol. Vet. 2008;17:87–92. doi: 10.1590/s1984-29612008000200005. [DOI] [PubMed] [Google Scholar]
- Ruiz de Ybanez M.R., Garijo M.M., Alonso F.D. Prevalence and viability of eggs of Toxocara spp. and Toxascaris leonina in public parks in eastern Spain. J. Helminthol. 2001;75:169–173. [PubMed] [Google Scholar]
- Ryan G.E. Helminth parasites of the fox (Vulpes vulpes) in New South Wales. Aus. Vet. J. 1976;52:126–131. doi: 10.1111/j.1751-0813.1976.tb05445.x. [DOI] [PubMed] [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Saunders G., Coman B., Kinnear J., Braysher M. Australian Government Publishing Service; Canberra: 1995. Managing Vertebrate Pests: Foxes. [Google Scholar]
- Schell S.C. W.C. Brown Co.; Dubuque, Iowa: 1970. How to Know the Trematodes. [Google Scholar]
- Schmidt G., Roberts L. Times Mirror/Mosby College Publishing; Missouri: 1985. Foundations of Parasitology. [Google Scholar]
- Shepherd, D.P., Beeston, G.R., Hopkins, A.J.M., 2001. Native vegetation in Western Australia. Technical Report 249. Department of Agriculture, Western Australia, South Perth.
- Sprent J.F.A., Barrett M.G. Large roundworms of dogs and cats: differentiation of Toxocara canis and Toxascaris leonina. Aus. Vet. J. 1964;40:166–171. [Google Scholar]
- Strahan R. Angus and Robertson; Sydney: 1983. The Australian Museum Complete Book of Australian Mammals. [Google Scholar]
- Stromberg B.E. Environmental factors influencing transmission. Vet. Parasitol. 1997;72:247–264. doi: 10.1016/s0304-4017(97)00100-3. [DOI] [PubMed] [Google Scholar]
- Vervaeke M., Dorny P., Bruyn L.D., Vercammen F., Jordaens K., Van Den Berge K., Verhagen R. A survey of intestinal helminths of red foxes (Vulpes vulpes) in Northern Belgium. Acta Parasitol. 2005;50:221–227. [Google Scholar]
- Wolfe A., Hogan S., Maguire D., Fitzpatrick C., Vaughan L., Wall D., Hayden T.J., Mulcahy G. Red foxes (Vulpes vulpes) in Ireland as hosts for parasites of potential zoonotic and veterinary significance. Vet. Rec. 2001;149:759–763. [PubMed] [Google Scholar]
- Yamaguti S. Interscience Publishers; New York, London: 1963. Systema Helminthum: Acanthocephala. [Google Scholar]


