Graphical abstract
Highlights
► We observed behaviour of uninfected mosquitoes and those infected with Hepatozoon parasites. ► We observed mosquito choice of infected and uninfected frogs and snakes. ► Host-seeking behaviour of infected Culex pipiens differed from uninfected mosquitoes. ► We report the first parasite-induced changes in host behaviour in this system.
Keywords: Hepatozoon, Culex, Parasite, Behaviour, Host-seeking, Manipulation
Abstract
Hepatozoon species are heteroxenous parasites that commonly infect the blood of vertebrates and various organs of arthropods. Despite their ubiquity, little is known about how these parasites affect host phenotype, including whether or not these parasites induce changes in hosts to increase transmission success. The objectives of this research were to investigate influences of the frog blood parasite Hepatozoon clamatae and the snake blood parasite Hepatozoon sipedon on host-seeking and host-choice behaviour of the mosquitoes Culex territans and Culex pipiens, respectively. During development of H. sipedon in C. pipiens, significantly fewer infected mosquitoes fed on uninfected snakes compared to uninfected mosquitoes. When H. sipedon was mature in C. pipiens, the number of infected and uninfected C. pipiens that fed on snakes was not significantly different. Higher numbers of mosquitoes fed on naturally infected snakes and frogs compared to laboratory-reared, uninfected control animals. However, experiments using only laboratory-raised frogs revealed that infection did not significantly affect host choice by C. territans. Behaviour of C. pipiens in the presence of H. sipedon may increase transmission success of the parasite and provide the first evidence of phenotypic changes in the invertebrate host of Hepatozoon parasites.
1. Introduction
Manipulation of host phenotype, including physiology and behaviour, is a widespread strategy employed by parasites to increase transmission success (Thomas et al., 2005; Lefèvre and Thomas, 2008). During development in haematophagous hosts, blood parasites could benefit by inhibiting host-seeking behaviour of the vector, a strategy that offers protection against death of both the invertebrate host, and thus the parasite, by predation or by defensive behaviour of vertebrate hosts (Klowden and Lea, 1979; Rivero and Ferguson, 2003; Parker et al., 2009). Conversely, mature, trophically transmitted blood parasites would benefit if conspicuousness of its blood-feeding vector results in increased frequency of ingestion by its vertebrate host (Parker et al., 2009).
Hepatozoon species of the phylum Apicomplexa are cosmopolitan, intracellular blood parasites of terrestrial vertebrates. These protozoa are transmitted to haematophagous arthropods through infected blood meals and are passed to vertebrates when they ingest infected arthropods, but not by infective bites of arthropods (Smith, 1996). Parasites of interest in this study are Hepatozoon clamatae, which infects erythrocytes of green frogs, Rana clamitans (Kim et al., 1998), and Hepatozoon sipedon, which infects erythrocytes of common garter snakes, Thamonophis sirtalis. Culex territans mosquitoes serve as natural definitive hosts for both parasites following ingestion of infected blood from frogs or snakes. Female C. territans, widespread through North America and Europe, feed readily on both snakes and frogs but prefer the blood of amphibians and share a close sympatric relationship with the green frog, R. clamitans (Bartlett-Healy et al., 2008). Culex pipiens, the most widespread mosquito species in the world (Barr, 1967) and the primary vectors of West Nile Virus (Fonseca et al., 2004), are generally considered to be ornithophilic but will feed on the blood of snakes and also serve as definitive hosts for H. sipedon. It is exceedingly difficult to establish a colony of C. territans in the laboratory during the majority of the year in temperate climates. Therefore, C. pipiens, which are available for purchase and easy to maintain as a colony throughout the year, are commonly used as experimental hosts for H. sipedon (Smith et al., 1994). H. sipedon requires a ranid frog as an additional vertebrate host, because these frogs serve as an intermediate host that eats mosquitoes and that is eaten by snakes (Smith et al., 1994).
H. clamatae and H. sipedon each require 30 days to mature to a transmissible stage in their definitive mosquito hosts (Smith et al., 1994; Kim et al., 1998). Predation by insectivorous green frogs is of primary concern for these Hepatozoon species due to the affinity of C. territans for amphibian blood and the nature of the diet of green frogs. Thus, these parasites would be well served to decrease host-seeking behaviour during development and to increase host-seeking behaviour when mature. In the latter scenario, behavioural manipulation that would increase conspicuousness of the mosquito to the frog may involve changing the direction of approach to a forward advance from the usual approach from the rear employed by C. territans (Crans, 1970), or may involve increasing persistence of feeding (see Anderson et al., 1999). Risky feeding behaviour by mosquitoes containing mature parasites may thus result in increase transmission of Hepatozoon species.
To increase transmission from a vertebrate host to a blood-feeding vector, parasites may act to increase the attractiveness of the vertebrate host to the biting invertebrate (Moore, 1993). Differential attractiveness of biting flies to individual vertebrate hosts varies with several parameters, including age, sex, size (Port and Boreham; 1980; Kelly, 2001), pregnancy (Ansell et al., 2006) and even alcohol consumption (Lefèvre et al, 2010). Certain degrees of attractiveness may be attributed to olfactory cues, such as increased CO2 output from larger hosts (Ansell et al., 2006), and odour profiles can be highly specific in attracting various species of mosquitoes (De Jong and Knols, 1995; Takken and Knols, 1999; Cooperband et al., 2008). Infections of various parasites in mammals are often accompanied by changes in host body odour (Prugnolle et al., 2009), including changes in emanations from the skin and volatiles exhaled in the breath (O’Shea et al., 2002). Additionally, infection can change the composition of epidermal microbial flora populations, consequently modifying host odours, and thus kairomone profiles (Braks et al., 1999; O’Shea et al., 2002; Prugnolle et al., 2009).
Due to trophic, as opposed to salivary, transmission of Hepatozoon species, there would not be any benefit to H. clamatae if mosquitoes acquire blood from uninfected frogs. Therefore, these parasites would experience increased transmission success if kairomone profiles of infected frogs were more attractive to C. territans. Kairomones may emanate directly from infected frogs or possibly through rectal secretions of blood meal plasma, known as pre-diuresis, by mosquitoes during feeding that could contain volatiles (Ahmadi and McClelland, 1985) of parasite origin.
This study focused on the behaviour of C. territans, C. pipiens and green frogs during infection with Hepatozoon species. Host-seeking behaviour, or responsiveness to hosts and feeding success, of C. territans infected with H. clamatae, and of C. pipiens infected with H. sipedon, was investigated both during parasite development and once the parasite reached maturity. Additionally, attraction of C. territans to frogs was assessed by comparing the number of mosquitoes that chose to land or feed on frogs infected with H. clamatae or uninfected frogs.
2. Materials and methods
2.1. Collection and care of frogs, snakes and mosquitoes
Adult green frogs, R. clamitans, were collected from a pond in Wolfville, Nova Scotia in June and July of 2010 (45.093671, −64.349713). Young, recently transformed green frogs, including several with tails, were collected from ponds in Kentville (45.087699, −64.465638) and Hantsport, NS (45.032592, −64.178331) in August, 2010. Frogs were transported to the Weston Animal Care Facility at Acadia University and maintained on a diet of mealworms (Tenebrio molitor) and crickets (Acheta domestica) obtained from a local pet store. Temperature in the amphibian room was maintained at 22 °C and 60% humidity on a 14:10 light:dark cycle.
Two wild-caught adults and two laboratory-raised eastern garter snakes, Thamnophis sirtalis sirtalis, infected with H. sipedon were used in this study. One wild-caught adult and one laboratory-raised garter snake were not infected with H. sipedon and were used as ‘uninfected snakes’. Snakes were maintained on diet of earthworms (Lumbricus terrestris). Temperature in the reptile room was maintained 24 °C and 50% humidity on a 14:10 light:dark cycle. Guidelines of the Canadian Council for Animal Care were followed during handling and maintenance of all frogs and snakes.
In late June through late August 2010 and 2011, larvae of C. territans were collected from various ponds in King’s County, Nova Scotia (45.087702, −64.465923; 45.093671, −64.349713; 45.031075, −64.415245). Larvae of C. territans were also collected from Merchant’s Millpond State Park, North Carolina (36.441723, −76.682167). All larvae were housed in Rubbermaid® tubs filled with a mixture of pond water and fresh water, and were fed Nutrafin® baby fish food. Adults were maintained on fresh water and 10% sucrose. Egg rafts and larvae of C. pipiens were purchased from Carolina Biological Supply (Burlington, North Carolina, USA) and maintained as previously described in this section. Following a blood meal, C. pipiens and C. territans were provided with moistened cotton pads as sites for oviposition. Temperature in the insectary was maintained at 25 °C and 50% humidity on a 14:10 light:dark cycle.
2.2. Blood collection and determination of parasitaemia
Blood was obtained from frogs by piercing the maxillary vein along the jaw line, anterior to the tympanum, with a 27 gauge, 0.5 inch needle (Forzán et al., 2012). Blood was obtained from snakes by clipping a small (2 mm) piece of tail with a razor blade. A total of 30 μL of blood were collected in a heparinised micro-hematocrit capillary tube and the wound was sprayed with Bactine® antiseptic spray (Bayer Health Care, Toronto, ON) before the animal was returned to its cage (Forzán et al., 2012). Blood was smeared across the microscope slide using another clean slide as a spreader, and allowed to dry. Smears were fixed and stained with Hema 3® (Fisher Scientific, Ottawa, ON) and slides were viewed using bright field microscopy. Approximately 10 000 cells were counted to determine the parasitaemia, a measure of intensity of infection calculated as the percentage of erythrocytes infected by gamonts of Hepatozoon species.
2.3. Inoculation of mosquitoes and frogs with H. clamatae
To infect mosquitoes that would later be fed to frogs, two green frogs naturally infected with H. clamatae were placed in a 30 × 30 × 30 cm Plexiglas feeding cage containing 20 adult C. territans. Frogs were placed in the cage at dusk and left for three hours. Blood-fed mosquitoes, identified by their swollen, red abdomens, were removed from the feeding cage, placed in a holding container and provided with water and 10% sucrose. Mosquitoes were maintained for 30 days until parasites were mature. Six metamorph green frogs, three males and three females, were inoculated by force-feeding with infected mosquitoes, washed down with phosphate buffered saline (PBS) (8.0 g NaCl/L, 0.20 g KH2PO4/L, 1.44 g Na2HPO4/L and 0.24 g KCl/L, pH to 7.4). Blood was obtained from each of the remaining frogs 35 days post inoculation and examined for H. clamatae in the same manner as previously described. Three out of six frogs became infected and were used in interrupted paired-choice and uninterrupted trials.
2.4. Host-seeking behaviour of infected and uninfected C. territans
During the day, with exposure to natural light, a green frog with moderate parasitaemia (1% to 2%) was introduced into a Plexiglas feeding cage containing five to ten female C. territans, starved of sugar for 12 h, for 2 h. Mosquitoes were used in these trials 5–7 days following eclosion. Trials were conducted during the day based on observations of C. territans in previous studies (Benach, 1970; Crans, 1970), and because preliminary trials in our lab indicated that mosquitoes fed most readily when exposed to natural light (data not shown). Following trials, mosquitoes were transferred to holding containers and provided with water and 5% sucrose. A wet cotton makeup sponge submerged in a small Petri dish was provided as an oviposition site. At 15 days post-feeding (PF), female mosquitoes containing developing oocysts of H. clamatae were transferred from holding containers to feeding cages. An uninfected green frog was introduced into the cage. Female mosquito behaviour was observed to note host-seeking and blood-feeding attempts. After 1 h, the frog was returned to the Animal Care Facility. Fed mosquitoes were transferred into a separate holding container, and unfed mosquitoes were placed back in the original holding container. At 30 days PF, an uninfected frog was introduced as above to observe the behaviour of female mosquitoes carrying mature oocysts of H. clamatae. Following observations, all mosquitoes from this trial were dissected to check for eggs in the ovaries or mature oocysts of H. clamatae in the Malpighian tubules. Control trials were also performed in the manner detailed above; however, an uninfected frog was used in the initial feeding trial. Uninfected mosquitoes were maintained in the same conditions as infected mosquitoes and all experiments were conducted in same way. Mosquitoes were dissected at the end of experiments to count eggs.
Behaviour of C. territans was observed during initial feeding experiments with an infected frog and during subsequent feeding experiments at 15 days and 30 days PF with uninfected frogs. Host-seeking behaviour was recorded as the occurrence of landing on a host and initiating a blood meal. During initial feedings (0 days PF), the number of mosquitoes that fed during the course of each trial was recorded. During feeding trials at 15 and 30 days PF, the number of female mosquitoes that approached and fed on the uninfected frog, as well as female mosquitoes that remained stationary or avoided the frog, was recorded.
2.5. Host-seeking behaviour of infected and uninfected C. pipiens
Trials with C. pipiens were conducted in the same manner as described for C. territans with the following modifications. C. pipiens were permitted to feed on infected or uninfected garter snakes in separate cages at the beginning of the trial. Mosquitoes were then exposed to an uninfected green frog or an uninfected garter snake at 15 days PF and 30 days PF. Mosquitoes were exposed to frogs for 2 h, the maximum time permitted as per animal care protocols, and to snakes for up to 12 h. A total of 10 mosquitoes were initially placed in a feeding cage during trials in which mosquitoes were exposed to green frogs, and a total of 30–40 female mosquitoes was initially placed in a feeding cage during trials in which mosquitoes were exposed to garter snakes. Mosquitoes were dissected at the end of experiments to count mature oocysts of H. sipedon in the haemocoel.
Behaviour of C. pipiens was not observed during exposure to snakes. Instead, responsiveness was measured as engorgement success, indicated by mosquitoes that had fed by the end of the trial.
2.6. Preference of mosquitoes for infected and uninfected green frogs: interrupted feeding, paired-choice trials
A total of 30 female C. territans, starved of sugar for 12 h, were placed in a feeding cage in a room with natural light entering through windows. The room was maintained at 24 °C and a relative humidity of 50%. Two wild-caught adult green frogs were placed in the cage on either side of a mesh net that extended half-way up the height of the cage. This arrangement allowed mosquitoes to fly over each side of the cage but prevented contact between frogs. When a mosquito landed on a frog and began to probe, it was considered to have made a choice, and the mosquito was aspirated from the cage and placed in a separate holding container. Two frogs of each of the following parasitaemias were used in these trials: uninfected (0%), low (0.1–1%), moderate (1–2%) and high (2–3%). Each trial was repeated three times for each of the following six pairs of frogs: high parasitaemia versus uninfected, moderate parasitaemia versus uninfected, low parasitaemia versus uninfected, high parasitaemia versus moderate parasitaemia, high parasitaemia versus low parasitaemia, and moderate parasitaemia versus low parasitaemia.
Paired- choice trials were repeated using experimentally infected green frogs described previously. Two infected frogs, including one female with 10% parasitaemia, and one male with 2% parasitaemia, were paired separately with two uninfected frogs, including one of each sex, and trials were repeated four times. All possible pairs of infected versus uninfected frogs were tested. The number of mosquitoes to land on each frog and the time for each mosquito to land was recorded.
2.7. Preference of mosquitoes for infected and uninfected green frogs: uninterrupted feeding, single-choice trials
A total of 15 female C. territans, starved of sugar for 12 h, were introduced into each of two feeding cages, one containing a frog infected with H. clamatae, and one containing an uninfected frog, during daylight hours, and cages were placed in natural light. Three infected frogs, two male (parasitaemia of 2% and 0.8%) and one female (parasitaemia of 10%), and three uninfected frogs, two female and one male, were used in these trials. A total of six replicates were completed for these trials.
Mosquitoes were permitted to feed to repletion on frogs, and the total number of mosquitoes that fed fully was recorded. The time for each mosquito to land, the time between bites of different mosquitoes, the time to appearance of blood in the abdomen, and the time to appearance of pre-diuresis was recorded. However, data concerning pre-diuresis could not be reliably collected and was not used in analysis. Trials lasted one hour or until the last mosquito had finished a blood meal, with a maximum of 90 min.
2.8. Statistical analyses
Statistical analyses were performed in R (R Development Core Team, 2010) and SAS (SAS Institute Inc., 2011).
Fisher’s exact test or Chi-Square test (for tables in which values were >5) were used to compare numbers between infected and control groups of C. territans or C. pipiens that did or did not feed at 15 days and 30 days PF. A Chi-Square Test was used to test if proportions of mosquitoes that fed on uninfected hosts and those that fed on infected hosts were statistically different.
A two-way ANOVA was used to test if the mean number of mosquitoes choosing to land on a frog was different for frogs of varying levels of parasitaemia. Mosquitoes that did not make a choice were removed from the analysis. The number of mosquitoes that chose a frog was used as the response variable and infection level was used as an explanatory variable.
A Welch Two-Sample T-Test was used to compare the number of mosquitoes that chose to feed on infected or uninfected frogs. A Wilcoxon Rank Sum test was used for uninterrupted feeding trials to test if the proportion of mosquitoes landing on infected frogs over time was significantly different from the proportion of mosquitoes landing on uninfected frogs over time. The same procedure was also used to test if the amount of time between a bite by one mosquito and a subsequent bite by another mosquito was significantly different in uninterrupted and interrupted trials.
3. Results
3.1. Host-seeking behaviour of infected and uninfected C. territans
Infected and uninfected (control) C. territans displayed low levels of host-seeking behaviour at both 15 and 30 days PF, as indicated by the low numbers of mosquitoes that fed at these times (Table 1). Statistically significant differences were not observed in the proportion of infected or uninfected mosquitoes that fed at 15 days PF (Fisher’s Exact Test, P = 0.12) or at 30 days PF (P = 1.00). Both groups of C. territans retained their eggs, despite being provided with a site for oviposition, and all mosquitoes in the infected group contained sporocysts of H. clamatae in their Malpighian tubules.
Table 1.
Number of infected and uninfected C. territans that fed on uninfected frogs at 15 days and 30 days post-feeding on infected or uninfected (control) frogs.
| 15 days Post-feeding |
30 days Post-feeding |
|||
|---|---|---|---|---|
| Number that fed on frogs | Number that did not feed | Number that fed on frogs | Number that did not feed | |
| Infected mosquitoes | 5 | 11 | 1 | 14 |
| Uninfected mosquitoes | 0 | 12 | 0 | 12 |
Statistically significant differences were not observed in the proportion of infected or uninfected mosquitoes feeding at 15 days PF or 30 days PF.
3.2. Host-seeking behaviour of infected and uninfected C. pipiens
C. pipiens did not respond to frogs at 15 days nor at 30 days PF (Table 2). However, a proportion of C. pipiens, in both infected and control groups, was responsive to uninfected snakes at 15 days and 30 days PF (Table 3). A significantly lower proportion of infected C. pipiens (31%) than uninfected mosquitoes (52%) obtained a second blood meal from snakes at 15 days PF (Chi-Square Test, , P = 0.05). A very low proportion of both infected and uninfected mosquitoes obtained a blood meal at 30 days PF (Table 3), and the difference in these proportions were not significant (P = 0.44). Infection of mosquitoes was confirmed at 30 days PF by presence of oocysts in the abdomen. Following blood meals, all C. pipiens included in analysis oviposited on moistened cotton pads.
Table 2.
Number of infected and uninfected C. pipiens that were responsive to green frogs at 15 days and 30 days post-feeding on an infected or uninfected (control) garter snake.
| 15 days Post-feeding |
30 days Post-feeding |
|||
|---|---|---|---|---|
| Number responsive | Number non-responsive | Number responsive | Number non-responsive | |
| Infected mosquitoes | 0 | 35 | 0 | 30 |
| Uninfected mosquitoes | 0 | 14 | 0 | 14 |
Neither infected nor uninfected mosquitoes responded to green frogs at 15 days or 30 days PF.
Table 3.
Number of infected and uninfected C. pipiens that fed on uninfected garter snakes at 15 days and 30 days post-feeding on an infected or uninfected (control) garter snake.
| 15 days Post-feeding |
30 days Post-feeding |
|||
|---|---|---|---|---|
| Number that fed on snakes | Number that did not feed | Number that fed on snakes | Number that did not feed | |
| Infected mosquitoes | 26 | 55 | 1 | 29 |
| Uninfected mosquitoes | 25 | 24 | 1 | 9 |
A significantly lower proportion of infected mosquitoes fed on snakes at 15 days PF than uninfected mosquitoes. A statistically significant difference was not observed in the proportion of infected or uninfected mosquitoes that fed on snakes at 30 days PF.
3.3. Proportions of mosquitoes feeding on infected and uninfected frogs and snakes
Analysis of data from several trials of initial feeding (0 days PF) revealed that the proportion of mosquitoes that initially fed, versus did not feed, on frogs infected with H. clamatae (62%) and snakes infected with H. sipedon (69%), compared to the proportion of mosquitoes that initially fed on uninfected frogs (37%) or uninfected snakes (40%), respectively, was significantly higher (Table 4; , P = 0.02 for frogs and , P < 0.0001 for snakes).
Table 4.
Numbers of C. territans and C. pipiens that initially fed on infected or uninfected frogs and snakes, respectively.
|
C. territans and frogs |
C. pipiens and snakes |
|||
|---|---|---|---|---|
| Number that fed on frogs | Number that did not feed | Number that fed on snakes | Number that did not feed | |
| Infected animals | 34 | 21 | 90 | 40 |
| Uninfected animals | 19 | 32 | 52 | 78 |
A significantly higher proportion of mosquitoes fed on infected green frogs and infected garter snakes than on uninfected frogs or uninfected snakes, respectively.
3.4. Preference of mosquitoes for infected and uninfected green frogs: interrupted feeding, paired-choice trials
Higher numbers of mosquitoes chose to land on wild-caught frogs with high intensity infections of H. clamatae when paired with frogs with moderate intensity infections or without infection (Fig. 1). However, similar numbers of mosquitoes chose to land on wild-caught frogs from the four other infection intensity combinations (Fig. 1). Overall, infection level had a weakly significant effect on the number of mosquitoes choosing to land on a frog (Two-Way ANOVA, F3 = 2.4, P = 0.09). Trials repeated with experimentally infected frogs revealed that infection level in a frog did not affect the number of mosquitoes that chose to land (Fig. 2; F2 = 0.17, P = 0.84).
Fig. 1.
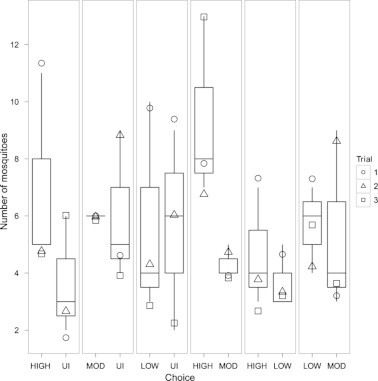
Number of mosquitoes that chose wild-caught frogs paired by different levels of infection. Each panel represents a pairing by infection level in green frogs. Each trial was repeated three times, and separate trials are shown by different shapes. Higher numbers of mosquitoes chose to land on wild-caught frogs with high infections of H. clamatae when paired with frogs with moderate infections or without infection. Infection level had a weakly significant effect on the number of mosquitoes that chose to land on a frog. UI = uninfected.
Fig. 2.
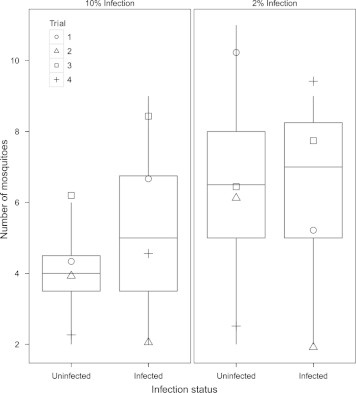
Number of mosquitoes that chose laboratory-raised frogs that were uninfected or experimentally infected. Each panel represents a pairing of infected or uninfected green frogs. Each pairing was repeated four times and each trial is represented by a different shape. Infection level did not have a significant effect on the number of mosquitoes choosing to land on a frog.
3.5. Preference of mosquitoes for infected and uninfected green frogs: uninterrupted feeding, single-choice trials
Similar numbers of mosquitoes fed on both infected (n = 39) and uninfected (n = 35) frogs over six trials (Welch’s Two Sample T-test, t74 = 0.5, P = 0.61). However, a significantly higher proportion of mosquitoes began to land on infected frogs later, or after approximately 20–30 min had elapsed in the trial, compared to the time to land on uninfected frogs (Fig. 3 top; Wilcoxon Rank Sum, W = 542, P = 0.04). Fortuitously, the time to land was also recorded in interrupted host choice trials, which allowed comparison between interrupted and uninterrupted feeding trials. Statistically significant differences in the time for mosquitoes to land on infected and uninfected frogs were not found in interrupted host choice trials (Fig. 3 bottom; W = 767, P = 0.36).
Fig. 3.
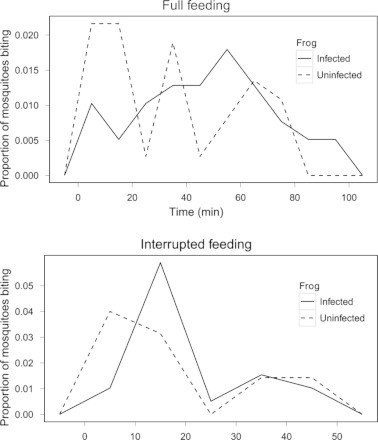
Time of mosquito biting activity on infected or uninfected frogs. Mosquitoes that fed on infected frogs are represented by the solid line, and those that fed on uninfected frogs are represented by the dashed line. The top figure represents the time for each mosquito to land in trials where mosquitoes were allowed to feed to repletion (i.e., uninterrupted trials). A significantly higher proportion of mosquitoes that fed on infected frogs began to land later in the trial, compared to mosquitoes feeding on uninfected frogs. The bottom figure represents the time for each mosquito to land in trials where mosquitoes were allow to land but were removed before feeding (i.e., interrupted trials). A significant difference in the time for mosquitoes to land on infected or uninfected frogs was not observed in interrupted trials.
A significantly longer interval elapsed between the bite of one mosquito and the bite of the subsequent mosquito when mosquitoes were allowed to feed, as opposed to when they were removed from the frog after landing (Fig. 4) (W = 992, P < 0.0001). Statistically significant differences in the interval between bites on infected frogs or uninfected frogs in either interrupted feeding trials (W = 445, P = 0.84) or uninterrupted trials (W = 529, P = 0.48) were not observed, and thus the total bites per trial, either with interrupted or uninterrupted feeding, were pooled for comparison.
Fig. 4.
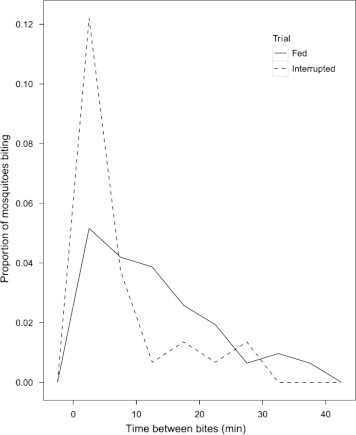
Time between mosquito bites on infected and uninfected frogs. Mosquitoes that were allowed to feed are represented by a solid line. Mosquitoes that were aspirated off the frog after landing are represented by the dashed line. A significantly longer amount of time passed between the bite of one mosquito and the bite of the following mosquito during trials in which mosquitoes were allowed to feed on a frog, compared to trials where mosquitoes were removed before feeding.
4. Discussion
To date, few studies have explored the effect of Hepatozoon species of parasites on both their vertebrate or invertebrate hosts. This study provides the first example of a measurable consequence to mosquito hosts during infection with these parasites, demonstrated through changes in behaviour.
A significant difference was not observed between numbers of infected or uninfected C. territans responding to hosts at 15 or 30 days PF; however, it is likely that results for C. territans were confounded by egg retention, in both infected and uninfected mosquitoes, which can inhibit host-seeking behaviour (Klowden, 1990). Thus, firm conclusions about the host-seeking behaviour of C. territans infected with H. clamatae cannot be drawn due to egg retention in the current study. Consequently, further analysis, such as power analysis to determine if any effect would emerge with a higher sample size, would likely not reveal useful information on the effects of H. clamatae on host-seeking behaviour of C. territans.
The lack of host-seeking response of C. pipiens to frogs is not surprising. Although frogs serve as the next host in the life cycle of H. sipedon following maturity in a mosquito, C. pipiens generally do not feed on frogs and thus we would not expect to observe a response to frogs in these trials, unless drastic parasite manipulation of behaviour had occurred.
However, host-seeking behaviour of infected C. pipiens was affected in the presence of garter snakes in the current study. A significantly lower proportion of infected than uninfected C. pipiens fed on garter snakes at 15 days PF when parasite development was occurring. At this time during infection, a decrease in host-seeking behaviour would be expected if the parasite is influencing the behaviour of its host (Egerter and Anderson, 1989; Parker et al., 2009). Host-seeking behaviour or feeding persistence of infected C. pipiens may be reduced, resulting in the fewer infected mosquitoes that fed. For example, decreases in the number of mosquitoes responding to hosts (Koella et al., 2002) as well as a decrease in feeding persistence (Anderson et al., 1999) are observed in Aedes aegypti infected with Plasmodium gallinaceum and Anopheles stephensi infected with Plasmodium yoelli nigeriensis, respectively. Decreased energy reserves in C. pipiens infected with H. sipedon could explain the decrease in host-seeking behaviour or feeding persistence. Biting persistence of and attack durations by Aedes species are significantly decreased in mosquitoes starved of sugar (Walker and Edman, 1985; Nasci, 1991). Each oocyst of H. sipedon grows to 250 μm, and several dozen may infect a mosquito (Smith et al., 1994), so should the growing oocysts of H. sipedon be depleting nutrient reserves of the mosquito, host-seeking behaviour and biting persistence could be reduced during parasite development. Other explanations include increased sugar feeding resulting in decreased blood feeding (Foster, 1995; Rivero and Ferguson, 2003). Sugar was removed for 12 h before mosquito behaviour was tested, potentially eliminating this as an explanation for the decrease in blood feeding; however, a decrease in host-seeking behaviour could be more pronounced if sugar was not removed. Conversely, illness-induced anorexia, or reduced feeding behaviour, could also explain low levels of blood feeding in infected C. pipiens compared to uninfected mosquitoes (Adamo, 2006).
Contrary to findings in the current study indicating that host-seeking of both infected and uninfected C. pipiens decreases at 30 days PF, both feeding persistence (Anderson et al., 1999) and the proportion of mosquitoes responding to hosts (Koella et al., 2002) increase in Anopheles species when Plasmodium species have reached transmissible stages. These strategies may increase salivary transmission of Plasmodium parasites; however, trophic transmission of Hepatozoon species may be facilitated by an overall decrease in activity levels that accompanies physiological aging, reflected as decreases of host-seeking behaviour. Frogs are drawn to both mosquitoes in flight and the waving of hind tarsi by mosquitoes at rest (Spielman and Sullivan, 1974). Should mosquitoes become less active as physiological age increases (Crans et al., 1976), or experience senescence of receptors (Seabrook et al., 1978; Den Otter et al., 1991) that may signal the presence of a predator, they may be more susceptible to predation by frogs. Indeed, daily survival rate and recapture rate of mosquitoes decrease with age (Harrington et al., 2001) and could be explained by increased predation. Such a natural physiological progression that accompanies aging may also benefit Hepatozoon species by increasing the risk of ingestion once parasites are mature.
The apparent preference by mosquitoes for infected hosts observed during initial feedings (0 days PF) of host-seeking trials (Table 4) prompted the hypothesis that frogs infected with H. clamatae are more attractive to C. territans. Children infected with gametocytes of Plasmodium falciparum are more attractive to mosquitoes, likely related to changes in odour during infection (LaCroix et al., 2005). Additionally, hamsters infected with Leishmania infantum attract higher numbers of sandflies and display different profiles of host volatiles (O’Shea et al., 2002). Thus, mosquito attraction to modified olfactory cues of infected frogs is a possible explanation for this observation.
However, when individual mosquito choice was tested by allowing C. territans to choose between wild-caught frogs with varying parasitaemia in the same cage, the effect of infection level on the number of mosquitoes that chose to land on a frog only trended towards significance. Although wild-caught frogs with high infections generally attracted higher numbers of mosquitoes, when trials were repeated with laboratory-raised frogs, a significant difference in the number of mosquitoes that chose infected or uninfected frogs was not observed. This analysis suggests that infection with H. clamatae alone does not attract higher numbers of mosquitoes.
The trending preference of mosquitoes to feed on wild-caught frogs with higher infections of H. clamatae may be explained by richer overall parasite communities in these frogs. Poulin (1995) provides evidence that richer parasite communities are linked to greater mean numbers of parasite individuals per host. Thus, the higher parasitaemia of H. clamatae in individual frogs in our study is suggestive of higher overall parasite species richness in these frogs, which may lead to behavioural (Edman et al., 1974; Day and Edman, 1983; Booth et al., 1993; Pederson and Fenton, 2007) or physiological (Kelly, 2001) changes to increase attraction and feeding success of mosquitoes (Klowden and Lea, 1979; Day et al., 1983; Moore, 1993; Kelly, 2001).
The low sample size of frogs used during these trials could increase the variability observed in mosquito choice during these trials. Individual frogs may vary in their attraction to mosquitoes, as is observed in mosquito attraction to various mammals (Kelly, 2001), and behaviour of frogs, such as activity level, could influence mosquito choice from day to day. A higher sample size of frogs would be beneficial to reduce the effect of the individual and highlight the effect of infection, but this was not possible in the given time frame owing to difficulty involved in experimental transmission of parasites among hosts.
It is important to note that during host-seeking trials in which significantly higher numbers of C. territans fed on infected frogs (Table 4), uninfected frogs were laboratory-raised juveniles and infected frogs were wild-caught adults. Captivity and diet can affect skin secretions of frogs (Smith et al., 2004; Williams et al., 2006), and age could affect production of other possible olfactory cues, such as pheromones (Poth et al., 2012). Thus, mosquito choice may be influenced by whether a frog is wild-caught or laboratory-raised, and adult or juvenile, rather than due to parasite influence.
Parasites entering a mosquito in a blood meal could release kairomones to host-seeking mosquitoes through rectal fluid (prediuresis) containing intact erythrocytes (Briegel and Rezzonico, 1985) or plasma contents (Roitberg et al., 2003) released by the mosquito as it feeds. Infected blood may contain elevated levels of components such as ammonia (Agarwal et al., 1997) that could then be released in prediuresis and act as an olfactory cue. However, during trials in which mosquitoes were permitted to feed to repletion, a significant difference in total numbers of mosquitoes feeding on infected or uninfected hosts was not observed. This result suggests that frogs infected with H. clamatae do not elicit increased feeding by larger proportions of mosquitoes.
Low peaks of feeding activity by C. territans exposed to infected frogs compared to mosquitoes exposed to uninfected frogs in the early part of uninterrupted trials may signal an initial reluctance to feed on infected frogs. However, this reluctance was subsequently overcome later in the trial, as indicated by similar overall numbers of mosquitoes feeding on both infected and uninfected frogs. Conversely, C. territans fed in similar patterns over time during interrupted trials. Again, a larger sample of frogs could provide clarity by reducing the effects of the individual frog; for example, a small sample size could include one or two active frogs whose individual behaviour, regardless of infection, skewed the data (Kelly, 2001).
During uninterrupted feeding trials, significantly more time elapsed between one mosquito bite and a subsequent bite by another mosquito than elapsed during interrupted trials. This may indicate that C. territans is more reluctant to feed on hosts on which conspecifics are already feeding. Increased numbers of mosquitoes on a host may increase the defensive behaviour of the host, which again may decrease feeding success or attraction of mosquitoes to a host (Edman et al., 1972). Aggregation effects of mosquitoes on attraction of mosquitoes to a host have thus far only been observed with Aedes species (Ahmadi and McClelland, 1985; Charlwood et al., 1995) and consistent conclusions on mosquito-mediated attraction cues have not been drawn (Charlwood et al., 1995). C. territans requires a prolonged interval to acquire a full blood meal (Crans, 1970) and the choice of an inactive host may reduce the likelihood of being knocked off by excess activity or defensive behaviour.
5. Conclusions
This study demonstrates that infection with H. sipedon affects the host-seeking behaviour, and potentially the transmission success of C. pipiens, and that H. clamatae may affect C. territans in a similar manner. The observed decrease in host-seeking behaviour of mosquito hosts during parasite development may increase transmission success of Hepatozoon species by protecting the host, and itself, from potentially fatal interactions with vertebrate hosts. Decreased host-seeking behaviour during this time may also result in reduced reproductive success, or fewer gonotrophic cycles, thus also affecting the fitness of the mosquito and the parasite. Additional effects of infection by Hepatozoon species remain to be elucidated and further studies could include measures of fitness, such as host longevity, as a function of the number of oocysts and the presence or absence of infection.
Acknowledgements
We wish to thank Chris Ogbuah and Chelsea Hammer for help in the collection and care of frogs and mosquitoes, Dave Shutler and Trevor Avery for help with statistical analyses, and Dawn Miner, Cate Little and Tanya Morse-Outhouse for the care of animals in the Animal Care Facility. This work was supported by a Natural Sciences and Engineering Research Council of Canada post-graduate scholarship to LVF and an Institutional Operating Fund from the Canadian Foundation of Innovation to TGS.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-Share Alike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Laura V. Ferguson, Email: lfergus9@uwo.ca.
N. Kirk Hillier, Email: kirk.hillier@acadiau.ca.
Todd G. Smith, Email: todd.smith@acadiau.ca.
References
- Adamo S. Comparative psychoneuro immunology: vevidence from the insects. Behav. Cog. Neurosci. Rev. 2006;5:128–140. doi: 10.1177/1534582306289580. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Tripathi L.M., Pandey V.C. Status of ammonia, glutamate, lactate and pyruvate during Plasmodium yoelii infection and pyrimethamine treatment in mice. J. Commun. Dis. 1997;3:235–241. [PubMed] [Google Scholar]
- Ahmadi A., McClelland G.A.H. Mosquito-mediated attraction of female mosquitoes to a host. Phys. Entomol. 1985;10:251–255. [Google Scholar]
- Anderson R.A., Knols B.G.J., Koella J.C. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology. 1999;120:329–333. doi: 10.1017/s0031182099005570. [DOI] [PubMed] [Google Scholar]
- Ansell J., Hamilton K.A., Pinder M., Walraven G.E.L., Lindsay S.W. Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Trans. Roy. Soc. Trop. Med. Hyg. 2006;96:113–116. doi: 10.1016/s0035-9203(02)90271-3. [DOI] [PubMed] [Google Scholar]
- Barr, A.R., 1967. Occurrence and distribution of the Culex pipiens complex. Bull. WHO 37, 293–296. [PMC free article] [PubMed]
- Bartlett-Healy K.W., Crans W., Gaugler R. Temporal and spatial synchrony of Culex territans (Diptera: Culicidae) with their amphibian hosts. J. Med. Entomol. 2008;45:1032–1038. doi: 10.1603/0022-2585(2008)45[1031:tassoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Benach J.L. Observations of a colony of Culex territans Walker. Proc. N. J. Mosqu. Exterm. Assoc. 1970;57:70–76. [Google Scholar]
- Booth D.T., Clayton D.H., Block B.A. Experimental demonstration of the energetic cost of parasitism in free-ranging hosts. Proc. Roy. Soc. Lond. B. Biol. 1993;253:125–129. [Google Scholar]
- Braks M.A.H., Anderson R.A., Knols B.G.J. Infochemicals in mosquito host selection: human skin microflora and Plasmodium parasites. Parasitol. Today. 1999;15:409–413. doi: 10.1016/s0169-4758(99)01514-8. [DOI] [PubMed] [Google Scholar]
- Briegel H., Rezzonico L. Concentration of host blood protein during feeding by anopheline mosquitoes (Diptera: Culicidae) J. Med. Entomol. 1985;22:612–618. doi: 10.1093/jmedent/22.6.612. [DOI] [PubMed] [Google Scholar]
- Charlwood J.D., Billingsley P.F., Hoc T.Q. Mosquito-mediated attraction of female European but not African mosquitoes to hosts. Ann. Trop. Med. Parasitol. 1995;89:327–329. doi: 10.1080/00034983.1995.11812962. [DOI] [PubMed] [Google Scholar]
- Cooperband M.F., McElfresh J.S., Millar J.G., Cardé R.T. Attraction of female Culex quinquefasciatus Say (Diptera: Culicidae) to odors from chicken feces. J. Insect Phys. 2008;54:1184–1192. doi: 10.1016/j.jinsphys.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Crans W.J. The blood feeding habits of Culex territans walker. Mosq. News. 1970;30:445–447. [Google Scholar]
- Crans W.J., Downing J.D., Slaff M.E. Behavioural changes in the salt marsh mosquito, Aedes sollicitans as a result of increased physiological age. Mosq. News. 1976;36:437–445. [Google Scholar]
- Day J.F., Edman J.D. Malaria renders mice susceptible to mosquito feeding when gametocytes are most infective. J. Parasitol. 1983;69:163–170. [PubMed] [Google Scholar]
- Day J.F., Ebert K.M., Edman J.D. Feeding patterns of mosquitoes (Diptera: Culicidae) simultaneously exposed to malarious and healthy mice, including a method for separating blood meals from conspecific hosts. J. Med. Entomol. 1983;20:120–127. doi: 10.1093/jmedent/20.2.120. [DOI] [PubMed] [Google Scholar]
- De Jong R., Knols B.G.J. Selection of biting sites on man by two malaria species. Experientia. 1995;51:80–84. doi: 10.1007/BF01964925. [DOI] [PubMed] [Google Scholar]
- Den Otter C.J., Tchicaya T., Schutte A.M. Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Phys. Entomol. 1991;16:173–182. [Google Scholar]
- Edman J.D., Webber L.A., Kale H.W. Effect of mosquito density on the interrelationship of host behaviour and mosquito feeding success. Am. J. Trop. Med. Hyg. 1972;21:487–491. doi: 10.4269/ajtmh.1972.21.487. [DOI] [PubMed] [Google Scholar]
- Edman J.D., Webber L.A., Schmid A.A. Effects of host defenses on the feeding pattern of Culex nigripalpus when offered a choice of blood sources. J. Parasitol. 1974;60:874–883. [PubMed] [Google Scholar]
- Egerter D.E., Anderson J.R. Blood-feeding drive inhibition of Aedes sierrensis (Diptera: Culicidae) induced by the parasite Lambornella clarki (Ciliophora: Tetrahymenidae) J. Med. Entomol. 1989;26:46–54. doi: 10.1093/jmedent/26.1.46. [DOI] [PubMed] [Google Scholar]
- Fonseca D.M., Keyghobadi N., Malcolm C.A., Mehmet C., Schaffner F., Mogi M., Fleischer R.C., Wilkerson R.C. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Forzán M.J.R., Vanderstichel V., Ogbuah C.T., Barta J.R., Smith T.G. Blood collection from the facial (maxillary)/musculo-cutaneous vein in true frogs (Family Ranidae) J. Wildl. Dis. 2012;148:176–180. doi: 10.7589/0090-3558-48.1.176. [DOI] [PubMed] [Google Scholar]
- Foster W.A. Mosquito sugar feeding and reproductive energetic. Annu. Rev. Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Harrington L.C., Buonaccorsi J.P., Edman J.D., Costera A., Kittayapong P., Clark G.G., Scott T.W. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidae) from Puerto Rico and Thailand. J. Med. Entomol. 2001;38:537–547. doi: 10.1603/0022-2585-38.4.537. [DOI] [PubMed] [Google Scholar]
- Kelly D.W. Why are some people bitten more than others? Trends Parasitol. 2001;17:578–581. doi: 10.1016/s1471-4922(01)02116-x. [DOI] [PubMed] [Google Scholar]
- Kim B., Smith T.G., Desser S.S. The life history and host specificity of Hepatozoon clamatae (Apicomplexa: Adeleorina) and ITS-1 nucleotide sequence variation of Hepatozoon species of frogs and mosquitoes from Ontario. J. Parasitol. 1998;84:816–820. [PubMed] [Google Scholar]
- Klowden M.J., Lea A.O. Effect of defensive host behaviour on the blood meal size and feeding success of natural populations of mosquitoes (Diptera: Culicidae) J. Med. Entomol. 1979;15:514–517. doi: 10.1093/jmedent/15.5-6.514. [DOI] [PubMed] [Google Scholar]
- Klowden M.J. The endogenous regulation of mosquito reproductive behaviour. Experientia. 1990;46:660–669. doi: 10.1007/BF01939928. [DOI] [PubMed] [Google Scholar]
- Koella J.C., Rieu L., Paul R.E.L. Stage-specific manipulation of a mosquito’s host-seeking behaviour by the malaria parasite Plasmodium gallinaceum. Behav. Ecol. 2002;13:816–820. [Google Scholar]
- LaCroix R., Mukabana W.R., Gougna L.C., Koella J.C. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:1590–1593. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre T., Thomas F. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infect. Genet. Evol. 2008;8:504–519. doi: 10.1016/j.meegid.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Lefèvre T., Gouagna L.-C., Dabiré K.R., Elguero E., Fontenille D., Renaud F. Beer consumption increases human attractiveness to malaria mosquitoes. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0009546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. Parasites and the behaviour of biting flies. J. Parasitol. 1993;79:1–16. [PubMed] [Google Scholar]
- Nasci R.S. Influence of larval and adult nutrition on biting persistence in Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 1991;28:522–526. doi: 10.1093/jmedent/28.4.522. [DOI] [PubMed] [Google Scholar]
- O’Shea B., Rebollar-Tellez E., Ward R.D., Hamilton J.G.C., El Naim D., Polwart A. Enhanced sandfly attraction to Leishmania-infected hosts. Trans. Roy. Soc. Trop. Med. Hyg. 2002;96:117–118. doi: 10.1016/s0035-9203(02)90273-7. [DOI] [PubMed] [Google Scholar]
- Parker G.A., Ball M.A., Chubb J.C., Hammerschmidt K., Millinski M. When should a trophically transmitted parasite manipulate its host? Evolution. 2009;63:448–458. doi: 10.1111/j.1558-5646.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- Pederson A.B., Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 2007;22:133–139. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Port G.R., Boreham P.F.L. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae complex (Diptera: Culicidae) Bull. Entomol. Res. 1980;70:133–144. [Google Scholar]
- Poth D., Wollenberg K.C., Vences M., Schulz S. Volatile amphibian pheromones: macrolides of mantellid frogs from Madagascar. Angewandte Chemie. 2012;51:2187–2190. doi: 10.1002/anie.201106592. [DOI] [PubMed] [Google Scholar]
- Poulin R. Phylogeny, ecology and the richness of parasite communities in vertebrates. Ecol. Monogr. 1995;65:283–302. [Google Scholar]
- Prugnolle F., Lefèvre T., Renaud F., Meller A.P., Misse D., Thomas F. Infection and body odours: volutionary and medical perspectives. Infect. Genet. Evol. 2009;9:1006–1009. doi: 10.1016/j.meegid.2009.04.018. [DOI] [PubMed] [Google Scholar]
- R Developmental Core Team, 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available from: <http://www.R-project.com>.
- Rivero A., Ferguson H.M. The energetic budget of Anopheles stephens infected with Plasmodium chabaudi: is energy depletion a mechanism for virulence? Proc. Roy. Soc. Lond. B Biol. 2003;270:1365–1371. doi: 10.1098/rspb.2003.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitberg B.D., Mondor E.B., Tyerman J.G.A. Pouncing spider, flying mosquito: blood acquisition increases predation risk in mosquitoes. Behav. Ecol. 2003;14:736–740. [Google Scholar]
- SAS Institute Incorporated, 2011. Base SAS® 9.3 Procedures Guide. Cary, NC.
- Seabrook W.D., Hirai K., Shorey H.H., Gaston L.K. Maturation and senescence of an insect chemosensory response. J. Chem. Ecol. 1978;5:587–594. [Google Scholar]
- Smith T.G., Desser S.S., Martin D.S. The development of Hepatozoon sipedon sp. nov. (Apicomplexa: Adeleina: Hepatozoidae) in its natural host, the Northern water snake (Nerodia sipedon sipedon), in the culicine vectors Culex pipiens and C. territans, and in an intermediate host, the Northern leopard frog (Rana pipiens) Parasitol. Res. 1994;80:559–568. doi: 10.1007/BF00933003. [DOI] [PubMed] [Google Scholar]
- Smith T.G. The genus Hepatozoon (Apicomplexa: Adeleina) J. Parasitol. 1996;1996(82):565–585. [PubMed] [Google Scholar]
- Smith B.P.C., Hayasaka Y., Tyler M.J., Williams B.D. β-Caryophyllene in the skin secretion of the Australian green tree frog, Litoria caerulea: an investigation of dietary sources. Aust. J. Zool. 2004;52:521–530. [Google Scholar]
- Spielman A., Sullivan J.J. Predation on peridomestic mosquitoes by hylid tadpoles on Grand Bahama Island. Am. J. Trop. Med. Hyg. 1974;23:704–709. doi: 10.4269/ajtmh.1974.23.704. [DOI] [PubMed] [Google Scholar]
- Takken W., Knols B.G. Odor-mediated behaviour of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Thomas F., Adamo S., Moore J. Parasitic manipulation: where are we and where should we go? Behav. Process. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Walker E.D., Edman J.D. The influence of host defensive behaviour on mosquito (Diptera: Culicidae) biting persistence. J. Med. Entomol. 1985;22:370–372. doi: 10.1093/jmedent/22.4.370. [DOI] [PubMed] [Google Scholar]
- Williams C.R., Smith B.P.C., Best S.M., Tyler M.J. Mosquito repellents in frog skin. Biol. Lett. 2006;2:242–245. doi: 10.1098/rsbl.2006.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]


