Graphical abstract
Keywords: Blood parasite, Body mass index, Breeding season, Haemoparasite, Hematology, Prevalence, Wild bird
Highlights
-
•
Variable prevalences of different haemoparasite species noted among passerine hosts.
-
•
Different foraging guilds associated with different haemoparasite infections.
-
•
Prevalence of Haemoproteus, Plasmodium, and Trypanosoma higher in breeding season.
-
•
PCV differences noted between bird species but no effect of haemoparasites on PCV or polychromasia.
-
•
Novel haplotypes detected and new geographic and host associations noted for seven haplotypes.
Abstract
The prevalence of five avian haemoparasite groups was examined for effects on health and associations with extrinsic factors. Overall, 786 samples were examined from six sites in two Georgia (USA) watersheds, during breeding and non-breeding periods in 2010 and 2011. Among the four most commonly infected species, Haemoproteus prevalence was significantly higher in Northern Cardinals (Cardinalis cardinalis) compared to Indigo Buntings (Passerina cyanea) and Tufted Titmice (Baeolophus bicolor) while prevalence in White-throated Sparrows (Zonotrichia albicollis) was significantly higher than in Indigo Buntings. Higher prevalence of Plasmodium was noted in Tufted Titmice and Northern Cardinals. While Leucocytozoon prevalence was highest in White-throated Sparrows, Trypanosoma prevalence was highest in Tufted Titmice. Interesting differences in infection probabilities were noted between foraging guilds with Haemoproteus associated with low-middle level strata and birds in the middle-upper strata were more likely to be infected with Plasmodium and Trypanosoma. In contrast, ground-foraging birds were more likely to be infected with Leucocytozoon. Breeding season was correlated with higher polychromasia counts and higher prevalence of Haemoproteus, Plasmodium and Trypanosoma. In addition, prevalence of infection with certain haemoparasite genera and packed cell volume (PCV) were different among host species. Body mass index was inversely correlated with prevalence of microfilaria infection but positively related to Haemoproteus infection. However, we found no relationship between PCV or polychromasia levels with haemoparasite infection. Molecular characterization of 61 samples revealed 19 unique Haemoproteus (n = 7) and Plasmodium (n = 12) haplotypes with numerous new host records. No differences were noted in haplotype diversity among birds with different migratory behaviors or foraging heights, thus additional studies are needed that incorporate molecular analysis, host biology, and vector biology into comprehensive models on parasite ecology. Detailed morphological examination of these parasites is also necessary to determine if closely related haplotypes represent single species or morphologically distinct, but closely related, haplotypes.
1. Introduction
Blood parasites have long been recognized as common in many wild bird species; however, their potential role as disease agents and their influence on avian ecology has been widely discussed (Loye and Zuk, 1991). The variation in the prevalence of blood parasites among species of birds has been used to test hypotheses about the effects of sexual selection, parental investment in disease resistance, and how vector abundance influences infection. In fact, infections with macro and micro-parasites are recognized as one of the most important selective pressures on birds (Seegar et al., 1976; Arriero et al., 2008). However, many factors (including environmental parameters that can influence vector abundance) related to this variation in prevalence are still poorly understood (Scheuerlein and Ricklefs, 2004). Although several studies have focused on describing the prevalence of avian parasitism in North American birds, most of the information available comes from controlled environments and/or with domestic bird species and most focus on describing prevalence alone and few examine differences in seasonality, geographic location, co-infections, or differences among free-ranging species (Greiner et al., 1975; Atkinson and van Riper, 1991; Deviche et al., 2005; Ricklefs et al., 2005; Hamer et al., 2013).
With some exceptions, haematozoa infections are rarely associated with significant mortality of wild birds infected with native parasites, but some studies suggest that these infections can have measurable effects on host fitness which may translate to population-level effects (Richner et al., 1995; Oppliger et al., 1996). For example, most passerines are altricial (are born less developed, highly dependent on progenitors), thus, factors that decrease progenitor performance would affect their offspring. Seasonal fluctuations of hormones (some with immunosuppressive activity) during breeding periods may make birds even more vulnerable to pathogens, especially if co-infected and/or exposed to other stressors, such as environmental extremes (Arriero et al., 2008). Blood parasites have been associated with higher levels of stress hormones (Arriero et al., 2008), increased disease susceptibility, and reduced survival rates because of increased predation (Atkinson et al., 2008). In addition, parasitized avian hosts have been demonstrated to have lower fat scores and mean body mass (Garvin et al., 2006). Parasitism has also been demonstrated to impair immunological response, decrease nest success and clutch size, decrease survival of yearlings, increase feather asymmetry and decrease expression of secondary sexual characteristics (Hõrak et al., 2001; Gilman et al., 2007).
This study focused on five groups of haemoparasites including three genera of Haemosporidae (Haemoproteus, Plasmodium and Leucocytozoon), larvae of filarioid nematodes (microfilaria), and Trypanosoma (Order Trypanosomatida). Parasites in the Order Haemosporida are intracellular protozoans found within blood cells and tissues of birds and although disease is uncommon in natural hosts, infections of unnatural hosts and/or susceptible age classes may result in death even in absence of clinical signs (Atkinson et al., 2008; Donovan et al., 2008). Trypanosoma spp. are extracellular haematozoa that are considered non-pathogenic, but can cause serious clinical signs in rare cases (Mandal et al., 2008). Filarioids are highly specialized nematode parasites of the tissues and interstitium of birds and other vertebrates and are often associated with non-specific clinical signs such as depression and weight loss (Bartlett, 2008). The clinical importance of filarial infection for wild birds seems to be associated with concomitant infections (Bartlett, 2008).
One of the potential effects to individuals infected with haemosporidian parasites is anemia caused by different mechanisms, including parasite-synthesized factors and increased erythrolysis and phagocytosis (Kocan and Clark, 1966; Kocan, 1968). In addition, the packed cell volume (PCV) (i.e., hematocrit) has been used as a variable to evaluate overall avian condition, although most studies have been descriptive (Dawson and Bortolotti, 1997). Studies investigating the direct relationship between PCV and haemoparasitism are scant and results are often contradictory (Ots and Hõrak, 1998; Booth and Elliott, 2003; Fair et al., 2007). Signs of a regenerative anemia, such as increased numbers of polychromatophilic erythrocytes, are also widely used in veterinary medicine in an attempt to measure bone marrow responses to a decrease in the numbers of erythrocytes from haemolysis, increased uptake of erythrocytes by the reticuloendothelial system, or other causes. Thus, measuring polychromasia may be a sensitive way of detecting evidence of previous anemia (Campbell, 2004a).
The ecology and transmission dynamics of haemoparasites can vary widely between locations and avian species because these parasites utilize haematophagous vectors with specific biting and habitat preferences (Martens et al., 1999), although the vector for many haemoparasite species is unknown. For example, habitat changes (e.g., agriculture) have the potential of changing the range, density and diversity of blood parasite vectors (Afrane et al., 2008; Lambin et al., 2010), which would alter the risk of birds becoming infected (Reiter and LaPointe, 2009). Specific portions of the annual cycle of avian hosts, such as the breeding period, can be associated with immunological changes that may influence the probability of infection and development of clinical disease from haemoparasites. For example, infections with Plasmodium spp. tend to decrease to chronic levels and remain dormant in the organs of hosts, leaving the blood circulation (van Riper et al., 1994), yet resume their haemoparasitic stage at the onset of the breeding season (Applegate and Beaudoin, 1970; Beaudoin et al., 1971).
The interaction between various pathogens within a host has received considerable recent attention, as it may influence host morbidity,mortality and population dynamics. Multiple infections of haemoparasites or concurrent infections with other pathogens (e.g. Salmonella spp.) may significantly change host and pathogen dynamics (del Cerro et al., 2010; Hamer et al., 2013). As part of a larger study to describe the spatial and temporal prevalence of infection of birds with Salmonella spp., we examined the interaction of haemoparasite prevalence and diversity with bird species, capture site, season, and Salmonella infection status. In order to better understand the sublethal effects of these parasites, we documented body mass index (BMI), and selected haematologic values (PCV and polychromasia). Specifically, we hypothesized that BMI and PCV would be lower in birds parasitized with a single or multiple parasite species and that polychromasia would be higher in infected birds.
2. Materials and methods
2.1. Study sites
Blood sampling was performed at six sites in two watersheds in Georgia, USA. Three sites were in Tift County in the Little River watershed, which drains Tifton, Georgia and from which Salmonella spp. is often recovered (Haley et al., 2009). This area is considered a key agricultural region, with nearly 40% in crops, 45% forested, and <15% pastures, urban areas and water. Topographically, it follows the characteristics of the coastal plain (heavy vegetation, flood plains, and stream channels). The altitude in this watershed varied from 700 to ∼5300 ft. Sampling stations were selected to include 1st to 4th order streams with assorted levels of flow during the course of the year. Three additional sites were located in the Oconee River watershed, which has an average altitude of 1000 ft, and is surrounded by a rural landscape, composed primarily of poultry, dairy and beef farming. Sampling stations were located along the North Oconee River and its tributaries (including Sandy Creek) in Madison, Jackson and Clarke Counties (Fig. 1).
Fig. 1.
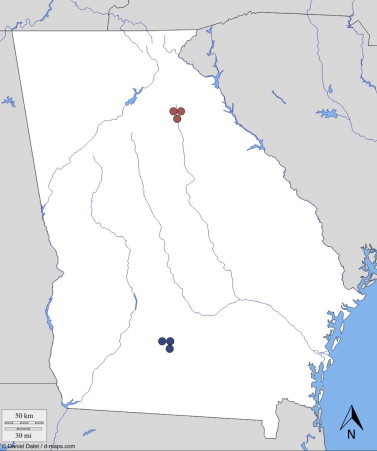
Map of Georgia (USA) indicating the location of the six sampling sites for identifying haemoparasite infections of birds in the northern and southern regions of the state.
2.2. Sampling methods
Sampling took place in two biologically-relevant seasons for birds: breeding (April–June) and non-breeding (September–March) in 2010 and 2011. Birds were captured using 12 and 18 m long, 38 mm mesh nylon mist nets, extended to a maximum height of 3 m from the ground. To control for body condition-biases in sampling (Gorney et al., 1999), we did not use food as lure for bird captures. Different numbers of mist nets were set, depending on the number of locations identified at a site with high bird activity, site capacity, and capture opportunity. Nets were checked every 15–20 min. All birds were identified to species, and banded with a butt-end metal Federal band. Each bird captured was physically examined and the following information was recorded: date of capture, gender (if possible), stage-based age (hatch year (HY) or adult), and standard morphometric measurements (body weight, wing cord, culmen, tarsus-metatarsus, tail length) (Pyle, 1997). The BMI was calculated by dividing body mass by the wing cord (Jones et al., 2009).
Blood was obtained with syringes with 27- or 29-gauge needles (BD insulin syringes; Becton Dickinson, 1 Becton Drive, Franklin Lakes, NJ 07417, USA) from the right jugular vein in songbirds, except for Columbiformes, from which blood was collected from the brachial vein. The volume of blood collected was never >1% of the total body weight. Two thin blood smears were immediately made from each sample and a portion of whole blood in heparin was used to calculate a PCV value by centrifuging a heparizined microhematocrit tube (StatSpin Microhematocrit tubes; StatSpin Technologies Inc., 60 Glacier Drive, Westwood, MA 02090, USA) at 10,000 rpm for 10 min. Remaining whole blood was frozen at −80 °C for future studies. Fecal samples were collected from disposable paper bags used to individually hold birds until they could be processed. For isolation of Salmonella, approximately 1 g of feces was enriched by inoculation into Dulcitol Selenite media (pre-prepared in house) and incubated at 41.5 °C for 18 h. Enrichment broths were streaked on Salmonella differential media XLT4, BGN and MacConkey agars (Remel, Lenexa, KS 66215, USA) followed by 37 °C incubation overnight. Colonies with appropriate colony morphology for the selective plate were isolated and further characterized with biochemical tests. A delayed-secondary enrichment was carried out for all samples that cultured negative after the first primary enrichment.
All birds were immediately released at the site of capture following sampling. The capturing, processing and handling procedures were review and approved by the University of Georgia IACUC committee (#A2010 08-159).
2.3. Light microscopy scanning methods
Blood smears were air-dried, fixed with 100% methanol, and stained using a modified Romanowsky staining technique (Diff Quick®, Jorgensen Laboratories, 1450 Van Buren Ave., Loveland, CO 80538, USA). The entire slide was scanned for microfilaria at 100×, and the monocellular layer was examined at 400× and 1000× (with oil immersion) for 10 min for haemoparasites, which were identified to genus based on morphology (Valkiunas, 2005). Polychromasia was determined by counting the number of polychromatophilic erythrocytes per 200 erythrocytes.
2.4. Molecular analysis
A subset (n = 113) of samples positive for haemotozoan parasites on blood smear was tested using a nested PCR that targets the cytochrome b (cyt b) gene. DNA was extracted from whole blood using DNeasy kit (Qiagen, Germantown, Maryland, USA) and amplification of partial cyt b gene was conducted using primers HaemNFI and HaemNR3 and HaemF and HaemR2 (Hellgren et al., 2004). Initially the PCR was conducted using a 55 °C annealing temperature; however, due to poor amplification of known positive samples, the annealing temperature was decreased to 50 °C. Amplicons were then sequenced bi-directionally at the University of Georgia Genomics Facility (Athens, Georgia, USA). Sequence chromatograms were analyzed in Sequencher v5.1 (Gene Codes Corp., Ann Arbor, MI, USA) and cleaned sequences were then aligned using MEGA v5.1 (Tamura et al., 2007) using the clustalW algorithm. Samples were characterized to genera using BLAST and a Neighbor Joining phylogenetic analysis. A minimum spanning tree was then calculated based on differences among haplotypes using Arlequin v3.11 (Excoffier et al., 2005). Only sequences that contained the full 479 bp between the HaemF and HaemR2 primers and did not contain any ambiguous bases where used for haplotype analysis. Comparison of number of haplotypes by site location (north vs. south), migratory status (resident vs. migratory), and foraging guild was done using Fisher’s exact test using R v2.15.
2.5. Data analysis
Although we captured and processed 53 species of birds, five were chosen for complete analysis because these species were most frequently captured and their life histories varied which may have altered exposure to vectors (e.g. foraging guild, migratory status) (Table 1). These target species were the Northern Cardinal (Cardinalis cardinalis), Tufted Titmouse (Baeolophus bicolor), White-throated Sparrow (Zonotrichia albicollis), Carolina Wren (Thryothorus ludovicianus) and Indigo Bunting (Passerina cyanea). For measures in which the covariate “species” was not statistically important, we ran the analysis with the total number of species that were captured. All analyses were performed using the statistical software SAS/STAT, Version 9.3 (SAS Institute, Inc., 2011). Due to insufficient sample size of hatch-year and after-hatch-year birds, these two age classes were grouped together and compared to adults. Birds were also placed in three different foraging guilds: ground, low-middle and middle-upper canopy according to Sibley (2003). A significance level of 0.05 was used for all analyses.
Table 1.
Characteristics of the five target species.
| Characteristics | Bird species |
||||
|---|---|---|---|---|---|
| Carolina Wren (Thryothorus ludovicianus) | Indigo Bunting (Passerina cyanea) | Northern Cardinal (Cardinalis cardinalis) | Tufted Titmouse (Baelophus bicolor) | White-throated Sparrow (Zonotrichia albicollis) | |
| Feeding guild | Ground | Low-mid | Low-mid | Mid-upper | Ground |
| Migratory status⁎ | Resident | Migratory | Resident | Resident | Migratory |
Note that resident birds can undergo local or regional movements whereas the two migratory species undergo long-distance movements between seasons.
Logistic regression was used to model the probability of infection from particular parasites based on all available information (e.g., species, age, sex, capture location, BMI and breeding season). All haemoparasites included in the study were used individually as dependent variables in separate logistic regression models. Polychromasia and PCV were not included in this analysis because of large number of missing values for these variables. An ordinal logistic regression was used to model the probability of different levels of infection. The response variable in this case is number of infections, with 3 categories: 0 for no infection, 1 for only one parasite present, and 2 for more than one parasite present. We used an ordinal logistic regression in order to model the ordinal response variable of infection, which records the number of infections of a single bird as 0, 1, or greater than 1. Two models are then created in this type of analysis (one for predicting the probability of having infections with two or more groups of parasites, and a second for predicting the probability of having infection with at least one parasite type). We worked under the proportional odds assumption that the odds ratio of having a higher level of infection for any particular predictor is the same for each of the two models.
To determine if there was any significant association between prevalence of infection with a particular haemoparasite and bird species (which includes migratory status and foraging guild), to test the association between prevalence of infection with each type of haemoparasite and breeding season, and to identify associations between prevalence of infection and the sites in which birds were sampled, we performed a Fisher’s exact test. Comparison of number of haplotypes by site location (North vs. South), migratory status (Resident vs. Migratory), and foraging guild was done using Fisher’s exact.
To determine if there was any significant relationship between the prevalence of infection with a particular haemoparasite and the PCV and polychromasia, and, whenever possible, whether age or gender influenced PCV or polychromasia, we performed t-tests. Before each t-test was run, a test of equal variances of the two groups (those with and without infection) was performed using a Folded F-test. Then, a method for the t-test (Pooled or Satterthwaite) was determined based on the outcome of the F-test. The pooled method for the t-test was used for the equal variance assumption, whereas the Satterthwaite method was used when unequal variances occurred (p-value of the Folded F-test <0.05).
To determine if there was any relationship between the species of the bird and PCV and polychromasia, we performed an ANOVA F-test as a preliminary test. If differences were noted, we conducted a multiple comparison test (Tukey’s test). We used a t-test to assess the associations between PCV, polychromasia, and BMI with breeding season and separately, with location. T-tests were also used to identify a relationship between prevalence of infection and BMI.
Lastly, we were interested in knowing whether there was any correlation between PCV and polychromasia, thus we utilized a Pearson correlation to examine this relationship.
3. Results
3.1. Prevalence of haemoparasites and Salmonella by species
A total of 786 birds of 53 species were sampled, including 381 birds of the five target species (Tables 2 and 3). All Carolina Wrens were negative for haemoparasites. Among the four target species infected with parasites, the prevalence of Haemoproteus was significantly higher in Northern Cardinals compared to Indigo Buntings (p = 0.0011) and Tufted Titmice (p = 0.0041) while prevalence in White-throated Sparrows was significantly higher than Indigo Buntings (p = 0.024) (Table 2). For Plasmodium, significantly higher prevalences were noted in Tufted Titmice and Northern Cardinals compared to the other species (all p < 0.032). Among the three infected species, Leucocytozoon prevalence was highest in White-throated Sparrows (p = 0.0075 and 0.0508 respectively). Prevalence of Trypanosoma was significantly higher in Tufted Titmice compared to the other three species (p = 0.027, 0.0061, and 0.0076). No differences were noted in the prevalence of microfilaria.
Table 2.
Haemoparasite results from five target species sampled in two regions of Georgia.
| Parasite | Bird species⁎ No. positive (%) |
||||
|---|---|---|---|---|---|
| Carolina Wren (Thryothorus ludovicianus) n = 33 | Indigo Bunting (Passerina cyanea) n = 58 | Northern Cardinal (Cardinalis cardinalis) n = 143 | Tufted Titmouse (Baelophus bicolor) n = 51 | White-throated Sparrow (Zonotrichia albicollis) n = 96 | |
| Haemoproteus | 0 | 8 (14)a | 55 (39)b | 8 (16)a | 29 (30)b |
| Plasmodium | 0 | 3 (5)b | 18 (13)a,b | 10 (20)a | 7 (7)b |
| Leucocytozoon | 0 | 0 | 7 (5)b | 2 (4)b | 15 (16)a |
| Microfilaria | 0 | 0 | 2 (1) | 1 (2) | 6 (6) |
| Trypanosoma | 0 | 1 (2)b | 5 (4)b | 8 (16)a | 2 (2)b |
Different letters, a, b, c and d, indicate significant differences (p < 0.05) in prevalence for the different haemoparasites.
Table 3.
Haemoparasite results from fifty non-target bird species sampled in Georgia.
| Bird species | n | Parasite species No. positive (%) |
|||||
|---|---|---|---|---|---|---|---|
| Haemoproteus | Plasmodium | Leucocytozoon | Microfilaria | Trypanosoma | |||
| Acadian Flycatcher | Empidonax virescens | 1 | –a | – | – | – | 1 (100) |
| American Goldfinch | Spinus tristis | 8 | 1 (13) | 1 (13) | 1 (13) | – | 1 (13) |
| American Robin | Turdus migratorius | 5 | – | 1 (20) | – | – | 1 (20) |
| Brown-headed Cowbird | Molothrus ater | 26 | 1 (4) | – | 1 (4) | 2 (8) | – |
| Blue Grosbeak | Passerina caerulea | 2 | – | – | – | – | – |
| Blue Jay | Cyanocitta cristata | 13 | – | 2 (15) | – | – | – |
| Brown Thrasher | Toxostoma rufum | 30 | 4 (13) | 2 7) | – | 1 (3) | 1 (3) |
| Carolina Chickadee | Poecile carolinensis | 11 | 5 (45) | 1 (9) | – | – | – |
| Chipping Sparrow | Spizella passerina | 6 | – | – | – | – | – |
| Common Ground-Dove | Columbina passerina | 19 | – | – | – | – | – |
| Common Yellowthroat | Geothlypis trichas | 7 | 2 (29) | – | – | – | – |
| Downy Woodpecker | Picoides pubescens | 2 | – | 1 (50) | – | – | – |
| Eastern Bluebird | Sialia sialis | 3 | 1 (33) | – | – | – | 1 (33) |
| Eastern Phoebe | Sayornis phoebe | 18 | – | 1 (6) | 1 (6) | – | – |
| Eastern Towhee | Pipilo erythrophthalmus | 18 | 3 (6) | 1 (6) | 1 (6) | 1 (6) | – |
| Eastern Wood-Pewee | Contopus virens | 2 | – | – | – | – | – |
| European Starling | Sturnus vulgaris | 5 | – | – | – | – | – |
| Field Sparrow | Spizella pusilla | 3 | – | – | – | – | – |
| Fox Sparrow | Passerella iliaca | 2 | – | – | – | – | – |
| Great Crested Flycatcher | Myiarchus crinitus | 3 | – | – | – | 1 (33) | – |
| Gray Catbird | Dumetella carolinensis | 7 | – | – | – | – | 1 (14) |
| Hermit Thrush | Catharus guttatus | 5 | – | – | – | – | – |
| House Finch | Carpodacus mexicanus | 13 | – | – | – | 1 (8) | – |
| House Sparrow | Passer domesticus | 69 | 1 (1) | 1 (1) | 3 (4) | – | – |
| Hooded Warbler | Setophaga citrina | 1 | – | – | – | – | – |
| Magnolia Warbler | Setophaga magnolia | 5 | – | 1 (20) | – | – | – |
| Myrtle Warbler | Setophaga coronata coronata | 11 | 3 (27) | – | – | 4 (36) | – |
| Northern Mockingbird | Mimus polyglottos | 17 | 6 (35) | – | – | – | – |
| Northern Parula | Setophaga americana | 1 | – | – | – | – | – |
| Northern Waterthrush | Parkesia noveboracensis | 2 | – | – | – | – | – |
| Prothonotary Warbler | Protonotaria citrea | 1 | – | – | – | – | – |
| Red-bellied Woodpecker | Melanerpes carolinus | 8 | 3 (38) | 2 (25) | – | – | – |
| Ruby-crowned Kinglet | Regulus calendula | 1 | – | – | – | – | – |
| Red-eyed Vireo | Vireo olivaceus | 1 | 1 (100) | – | – | – | – |
| Rock Pigeon | Columba livia | 1 | 1 (100) | – | – | – | – |
| Redwinged Blackbird | Agelaius phoeniceus | 5 | 1 (20) | 2 (40) | – | – | – |
| Savannah Sparrow | Passerculus sandwichensis | 14 | 2 (14) | 1 (7) | – | – | – |
| Slate-colored Junco | Junco h. hyemalis | 5 | – | – | 2 (40) | 1 (20) | – |
| Song Sparrow | Melospiza melodia | 5 | – | – | – | – | – |
| Sharp-shinned Hawk | Accipiter striatus | 3 | 1 (33) | – | – | – | – |
| Summer Tanager | Piranga rubra | 3 | 3 (100) | 1 (33) | – | – | – |
| Swamp Sparrow | Melospiza georgiana | 26 | 5 (19) | 2 (8) | – | – | – |
| Tennessee Warbler | Oreothlypis peregrina | 2 | – | – | – | – | – |
| White-eyed Vireo | Vireo griseus | 5 | 1 (20) | 1 (20) | – | – | – |
| Wood Thrush | Hylocichla mustelina | 2 | 1 (50) | – | 1 (50) | – | – |
| Western Palm Warbler | Setophaga palmarum palmarum | 3 | 1 (33) | – | – | – | – |
| Yellow-breasted Chat | Icteria virens | 1 | – | – | – | – | – |
| Yellow-bellied Sapsucker | Sphyrapicus varius | 4 | – | – | – | – | – |
All tested birds were negative.
No differences in prevalence were noted for any parasite species between male and female or between after hatch year (adult) and HY Northern Cardinals. Also, no differences were noted in prevalence of any parasite genus between adult and HY for Indigo Buntings and Tufted Titmice. For White-throated Sparrows, no differences between age classes were noted for Haemoproteus and Plasmodium, but significantly more adults (n = 67, 18%) were infected with Leucocytozoon compared to HY (n = 24, 0%).
Although limited analyses were conducted on the non-target species because of limited sample sizes or distribution among capture sites, great variability was noted in prevalence of infection for the five haemoparasites (Table 3). Prevalences of Haemoproteus and Plasmodium were generally higher and no single species of the non-target species was infected with all five groups of parasites.
Only five birds (one Northern Cardinal, one Brown-headed Cowbird (Molothrus ater), one European Starling (Sturnus vulgaris), one House Sparrow (Passer domesticus), and one Great-crested Flycatcher (Myiarchus crinitus) were positive for Salmonella spp. Of these, only the Great-crested Flycatcher was positive for a haemoparasite (microfilaria). Because of the low prevalence, no additional comparisons were possible.
3.2. Prevalence of haemoparasites by foraging guild
Interesting differences in probability of haemoparasite infections were noted between different foraging guilds (Table 4). Bird species foraging in the low-middle vertical stratum were more likely to be infected by Haemoproteus spp. compared to those in the middle-upper vertical stratum (p = 0.0301). In contrast, bird species foraging in the middle-upper vertical stratum were more likely to be infected with Plasmodium spp. compared to those that forage at ground level (p = 0.0058). Leucocytozoon infections were more likely in bird species foraging in the ground vertical stratum compared to other vertical stratum levels (p = 0.0062) and Trypanosoma infections were higher in bird species utilizing middle-upper vertical stratum compared to other strata (p = 0.0015). No differences were noted for microfilaria prevalence and foraging guilds (p = 0.0822).
Table 4.
Association of selected factors and infection with haemoparasites in wild birds from Georgia.
| Factor | n | Parasite species⁎ No. positive (%) |
|||||
|---|---|---|---|---|---|---|---|
| Haemoproteus | Plasmodium | Leucocytozoon | Microfilaria | Trypanosoma | |||
| Season | NonBreeding | 261 | 52 (20)a | 17 (7)a | 13 (5) | 7 (3) | 5 (2)a |
| Breeding | 120 | 48 (40)b | 21 (18)b | 11 (9) | 2 (2) | 11 (9)b | |
| Feeding guild | Ground | 129 | 29 (23)a,b | 7 (5)a | 15 (12)a | 6 (5) | 2 (2)a |
| Low-middle | 201 | 63 (31)b | 21 (11)a,b | 7 (4)b | 2 (1) | 6 (3)a | |
| Middle-upper | 51 | 8 (16)a | 10 (20)b | 2 (4)b | 1 (2) | 8 (16)b | |
| Migration status | Resident | 225 | 63 (28) | 27 (12) | 9 (4)a | 3 (1) | 13 (6) |
| Migrant | 154 | 27 (24) | 10 (7) | 15 (10)b | 6 (4) | 2 (2) | |
| Location | North | 244 | 57 (23) | 21 (9) | 18 (7) | 7 (3) | 15 (6)a |
| South | 137 | 43 (31) | 17 (12) | 6 (4) | 2 (2) | 1 (1)b | |
Different letters, a, b, c and d, indicate significant differences (p < 0.05) in prevalence for the different haemoparasites among the various factors included in the study.
3.3. Prevalence of haemoparasites by migratory behavior
No differences in prevalence were noted among those species that were either resident or migratory for Haemoproteus (p = 0.4084), Plasmodium (p = 0.0808), microfilaria (p = 0.1672), or Trypanosoma (p = 0.0747) (Table 4). However, the prevalence of Leucocytozoon spp. was higher for migratory birds compared to resident birds (p = 0.0311) (Table 4), although this difference resulted from the White-throated Sparrow data because all Indigo Buntings were negative.
3.4. Effects of haemoparasite infection, age, and gender on various hematology values
3.4.1. Packed cell volume (PCV)
Average PCV levels were similar between infected and non-infected birds of the same species for Haemoproteus (p = 0.4115; non-infected birds n = 184, mean PCV = 47%; infected birds n = 68, mean PCV = 46.4%), Plasmodium (p = 0.8486; non-infected birds n = 226, mean PCV = 46.9%; infected birds n = 26, mean PCV = 46.7%), Leucocytozoon (p = 0.9610; non-infected birds n = 239, mean PCV = 46.9%; infected birds n = 13, mean PCV = 46.8%), microfilaria (p = 0.2757, non-infected birds n = 244, mean PCV = 47%; infected birds n = 8, mean PCV = 44.8%), and Trypanosoma spp. (p = 0.3689; non-infected birds n = 243, mean PCV = 46.8%; infected birds n = 9, mean PCV = 48.6%).
Interestingly, there were different average PCV values for the target species (F = 22.13, p < 0.0001) (Fig. 2). Significantly higher average PCV values were detected for Indigo Buntings (52.4 ± 5.5) compared to other species, while Tufted Titmice (48.3 ± 3.4) had significantly higher average PCV than White-throated Sparrows (44.8 ± 4.2) and Carolina Wrens (42.2 ± 3.4), and Northern Cardinals (46.3 ± 5.6) had significantly higher average PCV than Carolina Wrens.
Fig. 2.
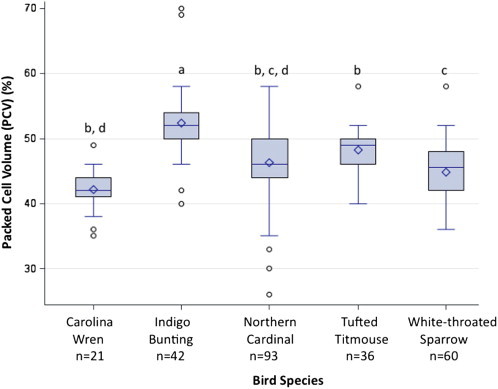
Average percent cell volume (PCV) values for five target bird species from Georgia (USA). Different letters indicate significant differences between bird species (p < 0.05).
An analysis of age showed that the mean value of PCV was significantly higher in HY birds (n = 61) compared to adult birds (n = 185) (F = 25.54, p = <0.0001); however, average PCV levels did not differ significantly between male (n = 59, mean 47.4 ± 6.24), female (n = 48, mean 46.4 ± 6.16), and unknown gender (n = 139, mean 46.9 ± 5.3; p = 0.6374). There was a negative correlation (r = −0.17) between PCV and BMI: as PCV decreased, BMI increased (p = 0.0068).
3.4.2. Association of polychromasia with haemoparasite infections, bird species, age, gender, BMI, and PCV
No significant differences in the average levels of polychromasia were found between birds infected and non-infected with Haemoproteus (p = 0.1731), Plasmodium (p = 0.3798), Leucocytozoon (p = 0.1923), microfilaria (p = 0.5394) or Trypanosoma (p = 0.0676). Interestingly, Northern Cardinals (n = 68, mean 4 ± 2.3) had a significantly higher average polychromasia level compared to White-throated Sparrows (n = 59, mean 2.9 ± 1.1) (p = 0.0043), yet no other species differences were noted for these levels.
Polychromasia levels were significantly higher in adult birds (n = 140, mean 3.59) compared to HY birds (n = 42, mean 3) (F = 4.19, p = 0.0084). While there was no difference in polychromasia levels between males (n = 44, mean 4 ± 2.2) and females (n = 32, mean 3.7 ± 2.2), they were higher than the unknown gender group (n = 105, mean 3.1 ± 1.1) (F = 4.64, p = 0.0108). Interestingly, although there was a negative correlation between PCV and BMI, we found a positive correlation (r = 0.15) between polychromasia and BMI (p = 0.04) with polychromasia levels increasing at higher BMI levels.
There was no correlation between the PCV and polychromasia in any bird species (all p > 0.05).
3.5. Effects of season on haemoparasite infections, hematology values and body mass index (BMI)
Prevalences of Haemoproteus Plasmodium, and Trypanosoma were higher during the breeding season compared to the non-breeding season, (p = <0.0001, 0.0015, and 0.0020, respectively) (Table 4). No differences in prevalence of infection with Leucocytozoon spp. (p = 0.1710) or microfilaria (p = 0.7255) were noted between breeding and non-breeding seasons.
Average PCV levels were similar in breeding (n = 58, mean 47) and non-breeding seasons (n = 194, mean 46.9) (F = 0, p = 0.9533); however, polychromasia was significantly higher during the breeding season (n = 68, mean 4.2) compared to the non-breeding season (n = 119, mean 3) (F = 21.11, p = 0.0002). Also, the mean BMI value for the five target species was significantly higher during the breeding season (n = 115, mean 0.4) compared to the non-breeding season (n = 259, mean 0.4) (F = 12.14, p = 0.0006).
3.6. Effects of sampling location on haemoparasite prevalence, BMI, and hematology values
No differences were noted for prevalence of infection between the six North and South Georgia sampling sites for Haemoproteus (p = 0.0910), Plasmodium (p = 0.2850), Leucocytozoon (p = 0.2803), and microfilaria (p = 0.4984) (Table 4). However, birds sampled in North Georgia had a higher prevalence of infection with Trypanosoma (p = 0.0138; Table 4).
The average PCV levels were significantly higher in South Georgia (n = 93, mean 49.2) compared to North Georgia sites (n = 159, mean 45.6) (F = 26.24, p = <0.0001); however, polychromasia did not significantly differ between the two locations (North = 3.3, n = 125 and South = 3.7, n = 62) (p = 0.250). As with PCV values, the average BMI indexes were significantly higher in South Georgia compared to North Georgia (0.4 [n = 132] vs. 0.4 [n = 242], respectively, F = 4.22, p = 0.0405).
3.7. Effects of haemoparasite infection on BMI
Intriguingly, the average BMI was significantly higher for birds infected with Haemoproteus spp. (n = 97, mean 0.4), compared to noninfected birds (n = 277, mean 0.4) (F = 12.8, p = 0.0004). However, no differences were noted in BMI values between infected and non-infected birds for Plasmodium spp. (p = 0.9532), Leucocytozoon spp. (p = 0.5302), microfilaria (p = 0.9203), or Trypanosoma spp. (p = 0.1561).
3.8. Overall predictors for haemoparasite infections
Two predictors, species and breeding season, were significant in the final model for Haemoproteus spp., whereas for Plasmodium spp., only the breeding season was significant (Table 5). In regards of Leucocytozoon spp., only the bird species was a significant predictor. Three variables (species, location and breeding season) were significantly associated with infection with Trypanosoma spp. (Table 5). Low prevalences precluded comparisons for microfilaria.
Table 5.
Overall predictors for haemoparasite infections in five target species.
| Parasite | Effect | df | Wald Chi-Square | p-Value |
|---|---|---|---|---|
| Haemoproteus spp. | Species | 3 | 12.1 | 0.0071 |
| Breeding season | 1 | 9.6 | 0.0019 | |
| Plasmodium spp. | Breeding season | 1 | 9.2 | 0.0024 |
| Leucocytozoon spp. | Species | 3 | 9.2 | 0.0272 |
| Trypanosoma spp. | Species | 3 | 11.5 | 0.0092 |
| Location | 1 | 4.9 | 0.0274 | |
| Breeding season | 1 | 10.4 | 0.0013 | |
After comparing breeding and non-breeding seasons with respect to the probability of infection with Haemoproteus spp., we determined that the odds of a bird being infected during the breeding season were 2.275 times than during the non-breeding season (Table 6). The odds of a bird being infected with Plasmodium during the breeding season were 2.882 times that of being infected during the non-breeding season. With respect to Leucocytozoon spp., the odds of Northern Cardinals being infected were 0.278 times those of White-throated Sparrows.
Table 6.
Odds of birds being infected with selected haemoparasites.
| Parasite | Comparison 1 | Comparison 2 | Estimate | SE | z Value | p-Value | Odds ratio |
|---|---|---|---|---|---|---|---|
| Haemoproteus | Breeding | Non-breeding | 0.8220 | 0.2650 | 3.10 | 0.0019 | 2.275 |
| Plasmodium | Breeding | Non-breeding | 1.0586 | 0.3490 | 3.03 | 0.0024 | 2.882 |
| Leucocytozoon | Northern Cardinal | Tufted Titmouse | 0.2318 | 0.8189 | 0.28 | 0.9568 | 1.261 |
| Northern Cardinal | White-throated Sparrow | −1.2805 | 0.4788 | −2.67 | 0.0205 | 0.278 | |
| Tufted Titmouse | White-throated Sparrow | −1.5122 | 0.7742 | −1.95 | 0.1240 | 0.220 | |
| Trypanosoma | Indigo Bunting | Northern Cardinal | −0.1286 | 1.1463 | −0.11 | 0.9995 | 0.879 |
| Indigo Bunting | Tufted Titmouse | −1.9636 | 1.1510 | −1.71 | 0.3204 | 0.140 | |
| Indigo Bunting | White-throated Sparrow | 0.2534 | 1.2918 | 0.20 | 0.9973 | 1.288 | |
| Northern Cardinal | Tufted Titmouse | −1.8350 | 0.6696 | −2.74 | 0.0312 | 0.160 | |
| Northern Cardinal | White-throated Sparrow | 0.3820 | 0.8906 | 0.43 | 0.9735 | 1.465 | |
| Tufted Titmouse | White-throated Sparrow | 2.2170 | 0.8398 | 2.64 | 0.0413 | 9.180 | |
| North GA | South GA | 2.3534 | 1.0673 | 2.21 | 0.0274 | 10.522 | |
| Breeding | Non-breeding | 2.0026 | 0.6214 | 3.22 | 0.0013 | 7.408 |
Since location (North vs. South) and breeding season were important factors for Trypanosoma prevalence, we determined the probability of infection with this haemoparasite between North and South Georgia, during breeding and non-breeding periods. The odds of a bird being infected with Trypanosoma in North Georgia were 10.522 times those of being infected in South Georgia and the odds of a bird being infected with Trypanosoma spp. during the breeding season were 7.408 times those of being infected during the non-breeding season (Table 6).
3.9. Multiple infections
Multiple infections were noted in three of the four target species that were infected with haemoparasites (Table 7). In Northern Cardinals, the most common combination was Haemoproteus and Plasmodium, which was also common in White-throated Sparrows (tied with Haemoproteus and Leucocytozoon co-infections). Co-infection with Plasmodium and Trypanosoma was most common in Tufted Titmice. Only a single bird (Northern Cardinal) was infected with three groups of parasites and a single White-throated Sparrow was infected with all five groups of parasites (Table 7).
Table 7.
Occurrence of single and multiple infections in the four target species infected with at least one species of haemoparasite.
| Number infected (% of infected birds) |
||||
|---|---|---|---|---|
| Indigo Bunting n = 12a |
Northern Cardinal n = 69 |
Tufted Titmouse n = 21 |
White-throated Sparrow n = 44 |
|
| Single parasite | ||||
| Haemoproteus only | 8 (67) | 39 (57) | 5 (24) | 18 (41) |
| Plasmodium only | 3 (25) | 7 (10) | 4 (19) | 2 (5) |
| Leucocytozoon only | 0 | 3 (4) | 2 (10) | 9 (20) |
| Microfilaria only | 0 | 0 | 0 | 3 (7) |
| Trypanosoma only | 1 (8) | 3 (4) | 2 (10) | 0 |
| Two parasites | ||||
| Haemoproteus + Plasmodium | 0 | 9 (13) | 1 (5) | 4 (9) |
| Haemoproteus + Leucocytozoon | 0 | 4 (6) | 0 | 4 (9) |
| Haemoproteus + microfilaria | 0 | 0 | 0 | 2 (5) |
| Haemoproteus + Trypanosoma | 0 | 2 (3) | 2 (10) | 0 |
| Plasmodium + microfilaria | 0 | 1 (1) | 1 (5) | 0 |
| Plasmodium + Trypanosoma | 0 | 0 | 4 (19) | 0 |
| Leucocytozoon + Trypanosoma | 0 | 0 | 0 | 1 (2) |
| Three parasites | ||||
| Haemoproteus + Plasmodium + microfilaria | 0 | 1 (1) | 0 | 0 |
| All five parasites | 0 | 0 | 0 | 1 (2) |
Number of birds infected with at least one parasite type.
Only single infections were noted in the Indigo Bunting (Table 7) which results in odds of this species having a multiple infections being lower than Northern Cardinals (0.364 times, p = 0.0391) and White-throated Sparrows (0.248 times, p = 0.0023), but not for Tufted Titmice (0.33 times, p = 0.0562).
We also established a comparison of breeding and non-breeding season with respect to the probability of higher levels of infection. The odds of a bird having a higher level of infection during the breeding season are 3.516 times those of being infected in non-breeding season (z = 5.16, p < 0.0001).
3.10. Molecular characterization
Partial cyt b gene was amplified from 87 of 113 (77%) samples screened by PCR. A total of 19 unique haplotypes of Haemoproteus (n = 7) and Plasmodium (n = 12) were detected from 61 birds (Fig. 3, Table 8). Two of the haplotypes (Haemoproteus SAIMEX01 and Plasmodium PADOM11) were detected in more than one species of birds (Fig. 3, Table 8); however, several of the previously detected haplotypes have been found in other bird species. Interestingly, based on a minimum spanning tree, several of the Haemoproteus haplotypes detected in White-throated Sparrows were closely related (Fig. 3). There were no differences in the number of unique haplotypes detected regardless of site location, migratory status, or foraging guild (Table 8). An additional 18 sequences were obtained from other birds but were not included in the haplotype analysis because they either had ambiguous bases (n = 11), only sequenced in one direction (n = 3), or sequenced in one direction but were identified as Leucocytozoon (n = 4). Interestingly, one Northern Cardinal had two distinct Plasmodium haplotypes (PADOM11 and SEIARU01) that were obtained from two independent PCR runs (one conducted with an annealing temperature of 55 °C and another at 50 °C), the chromatograms from these two PCR runs were bi-directional and did not contain any ambiguous bases.
Fig. 3.
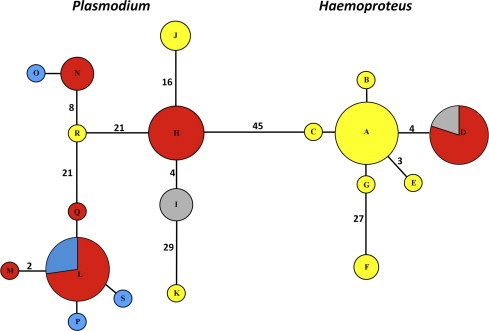
Minimum spanning network for Haemoproteus and Plasmodium mitochondrial DNA cytochrome b haplotypes detected in four species of passerines from Georgia (USA). Circles are drawn proportional to the frequency at which haplotypes were observed. Color represents the host species from which haplotypes originated: red for Northern Cardinal (Cardinalis cardinalis), blue for Indigo Bunting (Passerina cyanea), yellow for White-throated Sparrow (Zonotrichia albicollis), and grey for Tufted Titmouse (Baeolophus bicolor). A single mutation separates nodes unless explicitly indicated by number. Letters within each node refer to Table 8 which indicates the haplotype name, sampling location, and other factors associated with hosts.
Table 8.
Data on sampling location, foraging guild, and migratory status of the birds infected with the seven unique Haemoproteus haplotypes and 12 Plasmodium haplotypes.
| Parasite | Fig. 3 node⁎ | Haplotypes | n | Species‡ | Sampling location |
Migratory status |
Foraging height |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| North | South | Migratory | Resident | Ground | Low-mid | Mid-upper | |||||
| Haemoproteus | A | ZONALB01/GA/2011 | 10 | ZONALB | 10 | 0 | 10 | 0 | 10 | 0 | 0 |
| B | ZONALB02/GA/2011 | 1 | ZONALB | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| C | ZONALB03/GA/2011 | 1 | ZONALB | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| D | SAIMEX01† | 10 | BAEBIC, CARCAR | 5 | 5 | 0 | 10 | 0 | 8 | 2 | |
| E | ZONALB04/GA/2011 | 1 | ZONALB | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| F | ZONALB05/GA/2011 | 2 | ZONALB | 2 | 0 | 2 | 0 | 2 | 0 | 0 | |
| G | ZONALB06/GA/2011 | 1 | ZONALB | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| Total | 26 | 21 | 5 | 16 | 10 | 16 | 8 | 2 | |||
| Plasmodium | H | SEIARU01† | 7 | CARCAR | 2 | 5 | 0 | 7 | 0 | 7 | 0 |
| I | BAEBIC04/GA/2011 | 4 | BAEBIC | 4 | 0 | 0 | 4 | 0 | 0 | 4 | |
| J | BT7† | 3 | ZONALB | 3 | 0 | 3 | 0 | 3 | 0 | 0 | |
| K | TUMIG03† | 1 | ZONALB | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| L | PADOM11† | 11 | CARCAR, PASCYA | 3 | 8 | 3 | 8 | 0 | 11 | 0 | |
| M | CARCAR02/GA/2011 | 1 | CARCAR | 0 | 1 | 0 | 1 | 0 | 1 | 0 | |
| N | WW7† | 4 | CARCAR | 1 | 3 | 4 | 0 | 0 | 4 | 0 | |
| O | PASCYA01/GA/2011 | 1 | PASCYA | 0 | 1 | 1 | 0 | 0 | 1 | 0 | |
| P | PASCYA02/GA/2011 | 1 | PASCYA | 0 | 1 | 1 | 0 | 0 | 1 | 0 | |
| Q | CARCAR03/GA/2011 | 1 | CARCAR | 1 | 0 | 0 | 1 | 0 | 1 | 0 | |
| R | CATUST05† | 1 | ZONALB | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| S | PASCYA03/GA/2011 | 1 | PASCYA | 1 | 0 | 1 | 0 | 0 | 1 | 0 | |
| Total | 36 | 17 | 19 | 15 | 21 | 5 | 27 | 4 | |||
These letters refer to Fig. 3 to enable reader to visualize relationships of haplotypes. With the exception of haplotype H, all other haplotypes detected in this study were new host records.
These haplotypes had been previously reported in the MalAvi database (Bensch et al., 2009) which is available online at http://mbio-serv2.mbioekol.lu.se/Malavi/.
CARCAR, Northern Cardinal (Cardinalis cardinalis); PASCYA, Indigo Bunting (Passerina cyanea); ZONALB, White-throated Sparrow (Zonotrichia albicollis); and BAEBIC, Tufted Titmouse (Baeolophus bicolor).
4. Discussion
The five groups of parasites found in this study are all common parasites of wild birds, including the five target species (Love et al., 1953; Greiner et al., 1975; Garvin et al., 1993; Ricklefs and Fallon, 2002; Hartup et al., 2008; Martinsen et al., 2008; Hamer et al., 2013). The prevalences for the target species, except Carolina Wrens, were within ranges reported from previous surveys based on analysis of blood smears. The lack of haemoparasites in Carolina Wrens was surprising because infections have been reported previously in this species (Ricklefs and Fallon, 2002) and infections other close members of the Troglodytidae in the southeast USA have been reported (Garvin et al., 1993). It is possible that infections were missed due to low parasitemias or because of the lower sample size for this species. Among the non-target species, the very low prevalence in House Sparrows was similar to a previous study that found this invasive species has a low prevalence in non-endemic regions (Marzal et al., 2011). Our data were similar for the invasive European Starlings, although samples size was small (5) and additional sampling would be needed to see if they have also have experienced “enemy release” from their native parasites.
The initial intent of this study was to examine the effects of these haemoparasites on the health of birds and the effects of various factors on parasitism, and also to determine if there were interactions between haemoparasite infections and shedding of Salmonella. Salmonellosis can be a significant cause of morbidity and mortality for songbirds (Hernandez et al., 2012) and it is likely that there could be interactions between Salmonella and other pathogens. Despite the low prevalence of Salmonella infection detected in our sampled birds, we encourage future investigations to consider the impacts of co-infections on avian health as wildlife are rarely infected with any single agent.
Because all of the haemoparasites in this study are vector-borne, the probability of infection for birds is strongly related to vector presence, diversity, and abundance. Vector-host relationships have not been fully described for all North American haemoparasite species, but it is known that particular vector species partition vertical strata, which would make some birds more susceptible to transmission than others (Garvin and Greiner, 2003). In fact, the correlation between nesting height and prevalence of haemoparasite infection tends to be higher than the relationship between infection prevalence and plumage brightness (Garvin and Remsen, 1997; Deviche et al., 2005). We found that birds in the low-middle stratum were more likely to be infected with Haemoproteus spp. and birds in the middle-upper strata were more likely to be infected with Plasmodium and Trypanosoma. In contrast, birds foraging on the ground were more likely to be infected with Leucocytozoon spp. These findings contradict a recent study on the vertical distribution of ornithophilic bloodsucking Diptera (Culicidae, Simuliidae and Ceratopogonidae) conducted using bird-baited traps set at both ground and tree canopy levels (Cerny et al., 2011). Culex pipiens (Diptera: Culicidae), a vector of Plasmodium, was found to prefer ground-level habitats, whereas biting midges [Culicoides spp. (Diptera: Ceratopogonidae); vector for Haemoproteus] and Eusimulium angustipes (Diptera: Simuliidae; vector for Leucocytozoon) preferred the canopy (Cerny et al., 2011). Although Plasmodium prevalence, specifically in the southeastern USA, has been reported at approximately 10%, a correlation with foraging stratum has not previously been found (Greiner et al., 1975; Rohner et al., 2000). However, the positive relationship between Leucocytozoon spp. infection and ground foragers or nesters has been established and is related to black-fly (Simuliidae) activity (Fallis and Bennett, 1966; Rohner et al., 2000). However, this effect appears to vary by host species as ducks had a much higher prevalence of infection when nesting near the ground and the opposite was true for turkeys (Fallis and Bennett, 1966; Ricklefs, 2000; Forrester and Spalding, 2003). Interestingly, Great Horned Owls (Bubo virginianus) shift their roosting preferences from mid-canopy in winter to ground level in summer in what appears to be timed with the spring emergence of black flies which are most active in the mid-canopy (Rohner et al., 2000). In addition, some studies have failed to find any relationship betwen infection and foraging or nesting height (Ricklefs et al., 2005). The differences noted between the different studies could be explained by variation in haemoparasite haplotypes, most of which have unknown vectors. Co-infection with multiple haplotypes or sequential infections with different haplotypes are common in passerines (Ricklefs et al., 2005). Each of these haplotypes likely has a unique natural history with different vectors with variable host and habitat preferences. To address these discrepancies, a comprehensive study on haplotypes present in birds and vectors at different strata is needed.
According to our results, breeding season was correlated with a higher prevalence of infection with Haemoproteus spp., Plasmodium spp. and Trypanosoma spp. This is likely explained by either differences in vector abundance during the breeding season due to warmer temperatures or “spring relapse” of parasitemias due to hormonal changes or trade-offs between reproduction and parasite susceptibility (Allander, 1997; Stjernman et al., 2004). Importantly, seasonal variation can vary significantly between different species or haplotypes (e.g., P. relictum and P. circumflexum in Blue Tits (Parus caeruleus)) (Cosgrove et al., 2008). Similar to previous studies, we found no differences in the prevalence of most haemoparasites by migratory status, except for Leucocytozoon in White-throated Sparrows, which migrate north to breed where black flies are in high abundance (Garvin et al., 1993; Scheuerlein and Ricklefs, 2004). This finding was also supported by the absence of infection from HY White-throated Sparrows, suggesting that this species is infected on the breeding grounds. In contrast, we found no differences in Haemoproteus and Plasmodium prevalence between HY and adult birds indicating that transmission of these genera occurred at the breeding sites. We did find that more birds in northern Georgia were infected with Trypanosoma, but the reason for this difference is unknown. Generally, we would not expect significant differences in vector diversity or abundance related to climate between the two sites tested; however, the sites did differ in basic habitat type (one primarily agricultural, one riparian forest), which may alter vector presence. More likely, the majority of Trypanosoma infections were detected in Tufted Titmouse, and ∼82% of our samples from this species came from the northern sites in Georgia, likely influenced these results.
Causes and effects of the variation in the PCV of birds are difficult to explain but some researchers consider it a good measure of body condition and/or fitness (Brown, 1996; Hurtrez-Boussès et al., 1997; Potti et al., 1999), while others imply that the relationship does not exist, and that higher PCV levels may actually fluctuate with metabolism, workload or genetics (Dawson and Bortolotti, 1997; Cuervo et al., 2007; Potti, 2007). Significantly higher average PCV values were detected for Indigo Buntings compared to other species. This species is a long-distance migrant, flying over 1000 miles to its breeding grounds (Sibley, 2003). In some migratory species, PCVs can increase up to 60%, especially at the time of arrival to breeding grounds and post-nuptial molt (William, 2012). Thus, having a higher concentration of circulating erythrocytes may be a physiological adaptation to fulfill this demand (Potti, 2007).
The PCV of birds is known to be sensitive to environmental influences (Simon et al., 2005), which may vary with ambient temperature, presumably due to differences in demand of thermogenesis depending on the season (Carey and Morton, 1976). However, our results suggested that there was no difference between season and PCV. Reproduction is directly related to several endocrinological changes in birds. In terms of erythropoiesis, thyroid hormones enhance erythrocyte production and oxygen uptake, as triggered by exposure to light, affecting regulation of gonadal response to photoperiods (Yoshimura et al., 2003). Additionally, sex steroids are known to exert effects on erythropoiesis (Gordon et al., 1970), which may increase the release of immature erythrocytes to the peripheral circulation, perhaps explaining why polychromasia levels were higher during breeding season.
In wild birds, PCV is also known to fluctuate with gender, time of day, age, hydration and nutritional status and as a response to various environmental factors (Hodges, 1977; Rehder and Bird, 1983; Hawkey et al., 1984; Puerta et al., 1989; Bearhop et al., 1999; Reissig et al., 2002). Several studies have determined a positive correlation between PCV and age, but our results were not consistent with those findings (Palomeque et al., 1991). We could not find an explanation for why polychromasia levels would be higher in adults because, where this has been examined in other taxa (i.e., reptiles), the opposite is true (Campbell, 2004b). Equally, although we found no relationship between PCV and gender, polychromasia was higher in males, which may be explained by the effect of testosterone on the differentiation of stem cells into erythroid cells, which is documented in many vertebrate species.
Birds in this study had higher BMI during the breeding season, which is considered normal at the beginning of the breeding season, although fluctuations often occur during care of young (Alonso-Alvarez et al., 2002; Zillich and Black 2002). However, we did not find a significant correlation between BMIs and PCV. This agrees with other studies that have failed to detect a similar relationship (e.g. Dawson and Bortolotti, 1997).
We found few effects of parasitism on the health markers measured in this study. Our results did suggest that songbirds infected with microfilariae species are in poorer body condition. Although most filarial nematodes are not typically pathogenic for wild birds, some species can be lethal, causing chronic inflammation of pulmonary arteries, which may lead to impaired tissue oxygenation and decreased general metabolism (Bartlett, 2008). Interestingly, our data also showed that birds infected with Haemoproteus spp. had a higher average BMI than uninfected birds, but no differences were noted for other parasites. These findings are similar to previous studies conducted in European passerines, (Scheuerlein and Ricklefs, 2004) but are in contrast to others that found no differences (Bennett et al., 1988). In general, PCV is considered the most reliable measure of red blood cell numbers (Hodges, 1977; Campbell, 2004a) and values between 35% and 55% are considered normal for birds, whereas a PCV below 35% indicates anemia (Campbell, 2004a). Only one bird in this study, a Brown Thrasher (Toxostoma rufum), infected with Plasmodium spp. was anemic (PCV = 32%). Two additional birds were anemic (PCV = 34%) but neither was infected (Brown Thrasher and Northern Mockingbird (Mimus polyglottos). Equally, polychromasia levels did not help in identifying health effects of infected birds. In future studies, other haematological parameters, such as haemoglobin levels and mean corpuscular volume might prove to be more sensitive (Bearhop et al., 1999).
Although we only genetically characterized parasites from our four infected focal species, numerous novel Haemoproteus and Plasmodium haplotypes and seven previously identified haplotyes were detected. Among these seven, only one had been previously reported from one of our host species (SEIARU01 (Plasmodium) from Northern Cardinals). In our study, this particular haplotype was only found in Northern Cardinals but based on data from the MalAvi database, it has been reported from numerous bird species from North America including 13 species of Passeriformes and 3 species of Strigiformes and has been associated with mortality of a captive Green Jay (Cyanocorax yncas glaucescens) in Texas (Ferrell et al., 2007). The other six previously reported haplotypes were new host records for five Plasmodium haplotypes and one Haemoproteus haplotype. All but two (WW7 and CATUST05) of the Plasmodium haplotypes have been reported from numerous host species (n = 4–15) from North and South America, Europe, and Asia. The previously reported Haemoproteus haplotype (SIAMEX01) has been reported from the United States in eight species of native Passeriformes and was associated with mortality in a captive exotic Montezuma Oropendola (Psarocolius montezuma) and a lesser flamingo (Phoeniconaias minor) from a zoo in Texas (Ferrell et al., 2007). Interestingly, this haplotype had not previously been reported east of Missouri and Texas, thus, our report expands the host and geographic range of this potentially pathogenic Haemoproteus haplotype. Although the novel haplotypes detected in this study were only detected in a single species, additional studies are needed to determine the host specificity of these haplotypes because our sample size was limited and most haplotypes were infrequent; however, Haemoproteus haplotype ZONALB01/GA/2011 was common and only detected in White-throated Sparrows. Host specificity of avian haemosporidians has been shown in several other large-scale studies (Beadell et al., 2009; Hellgren et al., 2009). However, these studies and others have also shown several haplotypes are not host specific and can infect a broad host range (Ricklefs and Fallon, 2002; Belo et al., 2011). Interestingly, the White-throated sparrow, a migratory species, had the highest diversity of haplotypes; however, this species only migrates within North America, similar to the Indigo Bunting, which had a lower diversity of haplotypes. Because no differences were noted in haplotype diversity between our study sites, nor among bird species with different migratory behaviors or foraging heights, additional studies are needed that incorporate molecular analysis, host biology, and vector biology into comprehensive models of parasite ecology. Finally, careful and detailed morphological examination of these parasites is necessary to determine if closely related haplotypes in this study (e.g., A, B, C or N and O) represent single species or morphologically distinct, but closely related, haplotypes.
Acknowledgments
Partial funding was provided by an National Institutes of Health (National Institute of Allergy and Infectious Diseases) R15A1089565-01 grant. Additional support was provided by sponsorship of Southeastern Cooperative Wildlife Disease Study (SCWDS) by member fish and wildlife agencies and through Cooperative Agreements between SCWDS and Veterinary Services, United States Department of Agriculture-Animal and Plant Health Inspection Service and United States Geological Survey, United States Department of the Interior. Support from the states to SCWDS was provided in part by the Federal Aid to Wildlife Restoration Act (50 Stat. 917). We thank all members of the Hernandez Lab at the School of Forestry at the University of Georgia, who assisted with fieldwork and data collection, in addition to various undergraduate and graduate veterinary students for their assistance in laboratory methods and fieldwork. Lastly, we thank Dr. Carolyn Cray (University of Miami) for assistance with laboratory techniques.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Viviana González Astudillo, Email: vgonzalez01@unisalle.edu.co.
Sonia M. Hernández, Email: shernz@uga.edu.
Whitney M. Kistler, Email: whitney.kistler@gmail.com.
Shaun L. Boone, Email: sboone@uga.edu.
Erin K. Lipp, Email: elipp@uga.edu.
Sudip Shrestha, Email: sudip@uga.edu.
Michael J. Yabsley, Email: myabsley@uga.edu.
References
- Afrane Y.A., Little T.J., Lawson B.W., Githeko A.K., Yan G.Y. Deforestation and vectorial capacity of Anopheles gambiae giles mosquitoes in malaria transmission, Kenya. Emerg. Infect. Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander K. Reproductive investment and parasite susceptibility in the Great Tit. Funct. Ecol. 1997;11:358–364. [Google Scholar]
- Alonso-Alvarez C., Velando A., Ferrer M., Veira J.A.R. Changes in plasma biochemistry and body mass during incubation in the yellow-legged gull. Waterbirds. 2002;25:253–258. [Google Scholar]
- Applegate J.E., Beaudoin R.L. Mechanism of spring relapse in avian malaria: effect of gonadotropin and corticosterone. J. Wildl. Dis. 1970;6:443–447. doi: 10.7589/0090-3558-6.4.443. [DOI] [PubMed] [Google Scholar]
- Arriero E., Moreno J., Merino S., Martínez J. Habitat effects on physiological stress response in nestling Blue Tits are mediated through parasitism. Physiol. Biochem. Zool. 2008;81:195–203. doi: 10.1086/524393. [DOI] [PubMed] [Google Scholar]
- Atkinson C.T., van Riper C., III . Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoan, and Haemoproteus. In: Loye J.E., Zuk M., editors. Bird-Parasite Interactions: Ecology, Evolution and Behaviour. Oxford University Press; England: 1991. pp. 19–48. [Google Scholar]
- Atkinson C.T. Avian malaria. In: Atkinson C.T., Thomas N.J., Hunter D.B., editors. Parasitic Diseases of Wild Birds. Wiley-Blackwell Publishing; Ames: 2008. pp. 35–53. [Google Scholar]
- Bartlett C.M. Filarioid nematodes. In: Atkinson C.T., Thomas N.J., Hunter D.B., editors. Parasitic Diseases of Wild Birds. Wiley-Blackwell Publishing; Ames: 2008. pp. 439–462. [Google Scholar]
- Beadell J.S., Covas R., Gebhard C., Ishtiaq F., Melo M., Schmidt B.K., Perkins S.L., Graves G.R., Fleischer R.C. Host association and evolutionary relationships of avian blood parasites from West Africa. Int. J. Parasitol. 2009;39:257–266. doi: 10.1016/j.ijpara.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearhop S., Griffiths R., Orr K., Furness R.W. Mean corpuscular volume (MCV) as a measure of condition in birds. Ecol. Lett. 1999;2:352–356. [Google Scholar]
- Beaudoin R.L., Applegate J.E., Davis D.E., McLean R.G. A model for the ecology of avian malaria. J. Wildl. Dis. 1971;7:5–13. doi: 10.7589/0090-3558-7.1.5. [DOI] [PubMed] [Google Scholar]
- Belo N.O., Pinheiro R.T., Reis E.S., Ricklefs R.E., Braga E.M. Prevalence and lineage diversity of avian haemosporidians from three distinct cerrado habitats in Brazil. PloS One. 2011:e17654. doi: 10.1371/journal.pone.0017654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G.F., Caines J.R., Bishop M.A. Influence of blood parasites on the body mass of passeriform birds. J. Wildl. Dis. 1988;24:339–343. doi: 10.7589/0090-3558-24.2.339. [DOI] [PubMed] [Google Scholar]
- Bensch S., Hellgren O., Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Booth C.E., Elliott P.F. Hematologic responses to hematozoa in North American and neotropical songbirds. Comp. Biochem. Phys. A. 2003;133:451–467. doi: 10.1016/s1095-6433(02)00149-6. [DOI] [PubMed] [Google Scholar]
- Brown M.E. Assessing body condition in birds. In: Nolan V., Ketterson E.D., editors. Curr. Ornithol. Plenum Publishers; New York: 1996. pp. 67–135. [Google Scholar]
- Campbell T.W. Hematology of birds. In: Thrall M.A., editor. Veterinary Hematology and Clinical Chemistry. Blackwell Publishing; 2004. pp. 225–258. [Google Scholar]
- Campbell T.W. Hematology of reptiles. In: Thrall M.A., editor. Veterinary Hematology and Clinical Chemistry. Blackwell Publishing; 2004. pp. 259–276. [Google Scholar]
- Carey C., Morton M.L. Aspects of circulatory physiology of montane and lowland birds. Comp. Biochem. Physiol. A. 1976;51:61–74. doi: 10.1016/s0300-9629(76)80073-4. [DOI] [PubMed] [Google Scholar]
- Cerny O., Votypka J., Svobodová M. Spatial feeding preferences of ornithophilic mosquitoes, blackflies and biting midges. Med. Vet. Entomol. 2011;25:104–108. doi: 10.1111/j.1365-2915.2010.00875.x. [DOI] [PubMed] [Google Scholar]
- Cosgrove C.L., Wood M.J., Day K.P., Sheldon B.C. Seasonal variation in Plasmodium prevalence in a population of blue tits Cyanistes caeruleus. J. Anim. Ecol. 2008;77:540–548. doi: 10.1111/j.1365-2656.2008.01370.x. [DOI] [PubMed] [Google Scholar]
- Cuervo J.J., Møller A.P., de Lope F. Haematocrit is weakly related to condition in nestling Barn Swallows Hirundo rustica. Ibis. 2007;149:128–134. [Google Scholar]
- Dawson R.D., Bortolotti G.R. Are avian hematocrits indicative of condition? American kestrels as a model. J. Wildl. Manag. 1997;61:1297–1306. [Google Scholar]
- del Cerro S., Merino S., Martínez-de la Puente J., Lobato E., Ruiz-de-Castañeda R., Rivero-de Aguilar J., Martínez J., Morales J., Tomás G., Moreno J. Carotenoid-based plumage colouration is associated with blood parasite richness and stress protein levels in blue tits (Cyanistes caeruleus) Oecologia. 2010;162:825–835. doi: 10.1007/s00442-009-1510-y. [DOI] [PubMed] [Google Scholar]
- Deviche P., McGraw K., Greiner E.C. Interspecific differences in hematozoan infection in Sonoran desert Aimophila sparrows. J. Wildl. Dis. 2005;41:532–541. doi: 10.7589/0090-3558-41.3.532. [DOI] [PubMed] [Google Scholar]
- Donovan T.A., Schrenzel M., Tucker T.A., Pessier A.P., Stalis I.H. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J. Vet. Diagn. Invest. 2008;20:304–313. doi: 10.1177/104063870802000307. [DOI] [PubMed] [Google Scholar]
- Excoffier L., Laval G., Schneider S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Fair F., Whitaker S., Pearson B. Sources of variation in haematocrit in birds. Ibis. 2007;149:535–552. [Google Scholar]
- Fallis A.M., Bennett G.F. On the epizootiology of infections caused by Leucocytozoon simondi in Algonquin Park, Canada. Can. J. Zool. 1966;44:101–112. [Google Scholar]
- Ferrell S.T., Snowden K., Marlar A.B., Garner M., Lung N.P. Fatal hemoprotozoal infections in multiple avian species in a zoological park. J. Zoo Wildl. Med. 2007;38:308–316. doi: 10.1638/1042-7260(2007)038[0309:FHIIMA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Forrester D.J., Spalding M.G. University Press of Florida; Gainesville, FL: 2003. Parasites and Diseases of Wild Birds in Florida. [Google Scholar]
- Garvin M.C., Greiner E.C. Ecology of Culicoides (Diptera: Ceratopogonidae) in southcentral Florida and experimental Culicoides vectors of the avian hematozoan Heamoproteus danilewskyi Kruse. J. Wildl. Dis. 2003;39:170–178. doi: 10.7589/0090-3558-39.1.170. [DOI] [PubMed] [Google Scholar]
- Garvin M.C., Remsen J.V., Jr. An alternative hypothesis for heavier parasite loads of brightly colored birds: exposure at the nest. Auk. 1997;114:179–191. [Google Scholar]
- Garvin M.C., Remsen J.V., Jr., Bishop M.A., Bennett G.F. Hematozoa in passeriform birds from Louisiana. J. Parasitol. 1993;79:318–321. [PubMed] [Google Scholar]
- Garvin M.C., Szell C.C., Moore F.R. Blood parasites of nearctic-netropical migrant passerine birds during spring trans-gulf migration: impact on host body condition. J. Parasitol. 2006;92:990–996. doi: 10.1645/GE-758R.1. [DOI] [PubMed] [Google Scholar]
- Gilman S., Blumstein D.T., Foufopoulos J. The effect of hemosporidian infections on white-crowned sparrow singing behavior. Ethology. 2007;113:437–445. [Google Scholar]
- Gordon A.S., Zanjani E.D., Levere R.D., Kappas A. Stimulation of mammalian erythropoiesis by 5β-H steroid metabolites. PNAS. 1970;65:919–924. doi: 10.1073/pnas.65.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorney E., Clark W.S., Yom-Tov Y. A test of the condition-bias hypothesis yields different results for two species of sparrowhawks (Accipiter) Wilson Bull. 1999;111:181–187. [Google Scholar]
- Greiner E.C., Bennett G.F., White E.M., Coombs R.F. Distribution of the avian haematozoa of North America. Can. J. Zool. 1975;53:1762–1787. doi: 10.1139/z75-211. [DOI] [PubMed] [Google Scholar]
- Haley B.J., Cole D.J., Lipp E.K. Distribution, diversity, and seasonality of waterborne salmonellae in a rural watershed. Appl. Environ. Microbiol. 2009;75:1248–1255. doi: 10.1128/AEM.01648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer G.L., Anderson T.K., Berry G.E., Makohon-Moore A.P., Crafton J.C., Brawn J.D., Dolinski A.C., Krebs B.L., Ruiz M.O., Muzzall P.M., Goldberg T.L., Walker E.D. Prevalence of filarioid nematodes and trypanosomes in American robins and house sparrows in Chicago. Int. J. Parasitol. Parasites Wildl. 2013;2:42–49. doi: 10.1016/j.ijppaw.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartup B.K., Oberc A., Stott-Messick B., Davis A.K., Swarthout E.C.H. Blood parasites of house finches (Carpodacus mexicanus) from Georgia and New York. J. Wildl. Dis. 2008;44:469–474. doi: 10.7589/0090-3558-44.2.469. [DOI] [PubMed] [Google Scholar]
- Hawkey C.M., Hart M.G., Samour J.H. Age related haematological changes and haematological responses in Chilean flamingos (Phoenicopterus chilensis) Avian Pathol. 1984;13:223–229. doi: 10.1080/03079458408418526. [DOI] [PubMed] [Google Scholar]
- Hellgren O., Waldenström J., Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- Hellgren O., Pérez-Tris J., Bensch S. A jack-of-all trades and still a master of some: prevalence and host range in avian malaria and related blood parasites. Ecology. 2009;90:2840–2849. doi: 10.1890/08-1059.1. [DOI] [PubMed] [Google Scholar]
- Hernandez S.M., Keel K., Sanchez S., Trees E., Gerner-Smidt P., Adams J.K., Cheng Y., Ray A., III, Martin G., Presotto A., Ruder M.G., Brown J., Blehert D.S., Cottrell W., Maurer J.J. Epidemiology of a Salmonella enterica subsp. enterica serovar Typhimurium strain associated with a songbird outbreak. Appl. Environ. Microbiol. 2012;78:7290–7298. doi: 10.1128/AEM.01408-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges R.D. Normal avian (poultry) haematology. In: Archer R., Jeffcott L., editors. Comparative Clinical Haematology. Blackwell Scientific Publications; London: 1977. pp. 483–517. [Google Scholar]
- Hõrak P., Ots I., Vellau H., Spottiswoode C., Møller A.P. Carotenoid-based plumage coloration reflects haemoparasite infection and local survival in breeding great tits. Oecologia. 2001;126:166–173. doi: 10.1007/s004420000513. [DOI] [PubMed] [Google Scholar]
- Hurtrez-Boussès S., Blondel J., Perret P., Renaud F. Relationship between intensity of blowfy infestation and reproductive success in a Corsican population of blue tits. J. Avian Biol. 1997;28:267–270. [Google Scholar]
- SAS Institute Inc., 2011. SAS/STAT® 9.3 User’s Guide. Cary, NC: SAS Institute Inc.
- Jones K.A., Krebs J.R., Whittingham M.J. Heavier birds react faster to predators: individual differences in the detection of stalking and ambush predators. Behav. Ecol. Sociobiol. 2009;63:1319–1329. [Google Scholar]
- Kocan R.M. Anemia and mechanism of erythrocyte destruction in ducks with acute Leucocytozoon infections. J. Protozool. 1968;13:455–462. doi: 10.1111/j.1550-7408.1968.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Kocan R.M., Clark D.T. Anemia in ducks infected with Leucocytozoon simondi. J. Protozool. 1966;13:465–468. doi: 10.1111/j.1550-7408.1966.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Lambin E.F., Van Wambeke S.O., Linard C., Soti V. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int. J. Health Geogr. 2010;9:54. doi: 10.1186/1476-072X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love G.J., Wilkin S.A., Goodwin M.H., Jr. Incidence of blood parasites in birds collected in southwestern Georgia. J. Parasitol. 1953;39:52–57. [PubMed] [Google Scholar]
- Loye J.E., Zuk M. Oxford; New York: 1991. Bird-parasite interactions: Ecology, Evolution and Behavior. pp 4–15. [Google Scholar]
- Mandal M., Laha R., Sasmal N.K. First report of establishment of Trypanosoma evansi infection in pigeon nestlings (Columba livia) J. Parasitol. 2008;94:1428–1429. doi: 10.1645/GE-1509.1. [DOI] [PubMed] [Google Scholar]
- Martens P., Kovats R.S., Nijhof S., De Vries P., Livermore M.T.J., Bradley D.J., Cox J., McMichael A.J. Climate change and future populations at risk of malaria. Glob. Environ. Change. 1999;9:89–107. [Google Scholar]
- Martinsen E.S., Blumberg B.J., Eisen R.J., Schall J.J. Avian hemosporidian parasites from northern California oak woodland and Chaparral habitats. J. Wildl. Dis. 2008;44:260–268. doi: 10.7589/0090-3558-44.2.260. [DOI] [PubMed] [Google Scholar]
- Marzal A., Ricklefs R.E., Valkiūnas G., Albayrak T., Arriero E., Bonneaud C., Czirják G.A., Ewen J., Hellgren O., Hořáková D., Iezhova T.A., Jensen H., Križanauskienė A., Lima M.R., de Lope F., Magnussen E., Martin L.B., Møller A.P., Palinauskas V., Pap P.L., Pérez-Tris J., Sehgal R.N., Soler M., Szöllosi E., Westerdahl H., Zetindjiev P., Bensch S. Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS One. 2011;6:e21905. doi: 10.1371/journal.pone.0021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppliger A., Christe P., Richner H. Clutch size and malaria resistance. Nature. 1996;381:565. doi: 10.1038/381565a0. [DOI] [PubMed] [Google Scholar]
- Ots I., Hõrak P. Health impact of blood parasites in breeding great tits. Oecologia. 1998;116:441–448. doi: 10.1007/s004420050608. [DOI] [PubMed] [Google Scholar]
- Palomeque J., Pintó D., Viscor G. Hematologic and blood chemistry values of the Masai ostrich (Struthio camelus) J. Wildl. Dis. 1991;27:34–40. doi: 10.7589/0090-3558-27.1.34. [DOI] [PubMed] [Google Scholar]
- Potti J. Variation in the hematocrit of a passerine bird across life stages is mainly of environmental origin. J. Avian Biol. 2007;38:726–730. [Google Scholar]
- Potti J., Moreno J., Merino S., Frías O., Rodríguez R. Environmental and genetic variation in the haematocrit of fledgling pied flycatchers Ficedula hypoleuca. Oecologia. 1999;120:1–8. doi: 10.1007/s004420050826. [DOI] [PubMed] [Google Scholar]
- Puerta M.L., Nunoz-Pulido R., Huecas V., Abelenda M. Hematology and blood chemistry of chicks of white and black storks (Ciconia ciconia and Ciconia nigra) Comp. Biochem. Physiol. A. 1989;94:201–204. doi: 10.1016/0300-9629(89)90535-5. [DOI] [PubMed] [Google Scholar]
- Pyle P. Slate Street Press; Point Reyes Station, CA: 1997. Identification Guide to North American Birds, Part I: Columbidae to Ploceidae. 732 pp. [Google Scholar]
- Rehder N.B., Bird D.M. Annual profiles of blood pack cell volumes of captive American Kestrels. Can. J. Zool. 1983;61:2550–2555. [Google Scholar]
- Reissig E.C., Robles C.A., Sager R. Hematology and serum chemistry values of the lesser rhea (Pterocnemia pennata) raised in Patagonian farms (Argentina) J. Zoo. Wildl. Med. 2002;33:328–331. doi: 10.1638/1042-7260(2002)033[0328:HASCVO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reiter M.E., LaPointe D.A. Larval habitat for the avian malaria vector Culex quinquefasciatus (Diptera: Culicidae) in altered mid-elevation mesic-dry forests in Hawai’i. J. Vector Ecol. 2009;34:208–216. doi: 10.1111/j.1948-7134.2009.00028.x. [DOI] [PubMed] [Google Scholar]
- Richner H., Christe P., Oppliger A. Paternal investment affects prevalence of malaria. Proc. Natl. Acad. Sci. 1995;92:1192–1194. doi: 10.1073/pnas.92.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E. The relationship between local and regional species richness in birds of the Caribbean Basin. J. Anim. Ecol. 2000;69:1111–1116. [Google Scholar]
- Ricklefs R.E., Fallon S.M. Diversification and Host switching in avian malaria parasites. Proc. R. Soc. Lond. B Biol. Sci. 2002;269:885–892. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E., Swanson B.L., Fallon S.M., Martínez-Abraín A., Scheuerlein A., Gray J., Latta S.C. Community relationships of avian malaria parasites in southern Missouri. Ecol. Monogr. 2005;75:543–559. [Google Scholar]
- Rohner C., Krebs C.J., Hunter D.B., Currie D.C. Roost site selection of great horned owls in relation to black fly activity: an anti-parasite behavior? Condor. 2000;102:950–955. [Google Scholar]
- Scheuerlein A., Ricklefs R.E. Prevalence of blood parasites in European passeriform birds. Proc. Biol. Sci. 2004;271:1363–1370. doi: 10.1098/rspb.2004.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegar S.W., Everett S.L., Sladen W.J.L., Trpis M. A mallophaga, Trinoton anserinum, as a cyclodevelopmental vector for a heartworm parasite of waterfowl. Science. 1976;194:739–741. doi: 10.1126/science.982042. [DOI] [PubMed] [Google Scholar]
- Sibley D.A. Knopf Doubleday Publishing Group; Garden City, NY: 2003. The Sibley Field Guide to Birds of Eastern North America. 432 pp. [Google Scholar]
- Simon A., Thomas D.W., Bourgault P., Blondel J., Perret P., Lambrechts M.M. Between-population differences in nestling size and hematocrit level in blue tits (Parus caeruleus): a cross-fostering test for genetic and environmental effects. Can. J. Zool. 2005;83:694–701. [Google Scholar]
- Stjernman M., Raberg L., Nilsson J.-A. Survival costs of reproduction in the blue tit (Parus caeruleus): a role for blood parasites? Proc. Biol. Soc. 2004;271:2387–2394. doi: 10.1098/rspb.2004.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Valkiunas G. CRC Press; Boca Raton, FL: 2005. Avian Malaria Parasites and Other Haemosporidia. 935 pp. [Google Scholar]
- van Riper C., III, Atkinson C.T., Seed T.M. Plasmodia of birds. In: Kreier J.P., editor. Parasitic Protozoa. Academic Press; San Diego, CA: 1994. pp. 73–140. [Google Scholar]
- William T.D. Princeton University Press; New Jersey, USA: 2012. Physiological Adaptations for Breeding in Birds. [Google Scholar]
- Yoshimura T., Yasuo S., Watanabe M., Ligo M., Yamamura T., Hirunagi K., Ebhihara S. Light – induced hormone conversion of T4 and T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- Zillich U., Black J. Body mass and abdominal profile index in captive Hawaiian Geese. Wildfowl. 2002;53:67–77. [Google Scholar]


