Graphical abstract
Keywords: Cost of parasites, Lifespan, Meta-regression, Virulence
Highlights
-
•
A meta-analysis of the effects of parasites was conducted.
-
•
Significant effects of parasites at the population level were found.
-
•
A meta-regression of effect sizes on life-history traits was also done.
-
•
Host lifespan was found to correlate significantly with parasite virulence.
Abstract
Parasites are considered drivers of population regulation in some species; unfortunately the research leading to this hypothesis has all been conducted on managed populations. Still unclear is whether parasites have population-level effects in truly wild populations and what life-history traits drive observed virulence. A meta-analysis of 38 data sets where parasite loads were altered on non-domesticated, free-ranging wild vertebrate hosts (31 birds, 6 mammals, 1 fish) was conducted and found a strong negative effect of parasites at the population-level (g = 0.49). Among different categories of response variables measured, parasites significantly affected clutch size, hatching success, young produced, and survival, but not overall breeding success. A meta-regression of effect sizes and life-history traits thought to determine parasite virulence indicate that average host life span may be the single most important driver for understanding the effects of parasites. Further studies, especially of long-lived hosts, are necessary to prove this hypothesis.
1. Introduction
A central goal of population ecology is to identify factors controlling population dynamics. In wild populations, predation and competition are well studied, with some theoretical and empirical investigations focusing on the effects of parasites. Population regulation by parasites has been identified in Red Grouse Lagopus lagopus scoticus (Hudson et al., 1998), Svalbard Reindeer Rangifer tarandus platyrhnchus (Albon et al., 2002) and Soay Sheep Ovis aries (Gulland, 1992); unfortunately, these examples represent managed populations, and therefore may not reflect true effects of parasites on wild populations. Thus, the question remains—are parasites significant drivers of population-level effects and what host life-history traits drive observed virulence (sensu lato Casadevall and Pirofski, 1999—the capacity of a parasite to cause damage to a host)?
The modern view of parasitism is predicated on the assumption that ‘every parasitic organism… imposes a cost on its host’ because resources, however slight, are being diverted from host to parasite (Combes, 2005). These costs can be couched in two evolutionary trajectories: (1) the ‘mutual aggression model, (Holmes, 1983) which suggests that parasites evolve to be as virulent as possible, and thus are a primary regulatory force; and (2) the ‘prudent parasite model’ (Holmes, 1983; Renaud and de Meeüs, 1991) which suggests that parasites evolve towards a balance between short- and long-term needs conferring a range of benefits to the infected host that may or may not offset the costs (Michalakis et al., 1992; Schmidt-Hempel, 2003).
Several researchers have argued that the only way to assess the true effects of parasites is by altering the parasite population of the host in situ (Møller, 2005). Alterations of parasite loads are easy to effect in domestic and laboratory animals, and even wild animals in the laboratory (Diamond, 1983; McCallum, 1995). However, relatively little parasite work on wild, free-ranging hosts incorporates this technique due to logistical difficulties surrounding field work and obtaining sufficient sample sizes to detect differences between infected and non-infected hosts. Therefore, much ecological work on the effects of parasites ends up being correlative (Poulin, 2007a). It is unclear if the differences detected between parasitized and non-parasitised hosts are due to indirect effects or pre-existing differences (i.e., prior to infection; Bize et al., 2008 or host-quality; Lailvaux and Kasumovic, 2011). Field experimentation is necessary to quantify actual costs of parasites on hosts due to the many problems associated with extrapolating laboratory results on individuals or populations to real effects in the field (Seitz and Ratte, 1991).
In order to understand if parasites are truly a driver of host populations, reviews of the effects of parasites to wild hosts need to be conducted. Reviews to date of both observational and experimental work on the cost of parasites to wild hosts (birds, mammals, fish and insects: Lehmann, 1993; birds: Møller, 1997; birds and mammals: Tompkins and Begon, 1999; mammals: Irvine, 2006) have implied that parasites are costly, but the implications of that cost are unreliable, due to the methods used to synthesize results (Stewart, 2010). A recent meta-analytical synthesis of parasite induced mortality of nestlings showed an overall small effect (12% mean parasite-induced mortality, range 0–89%, n = 117), with parasite-induced mortality determined by latitude, nesting site, probability of host survival and parasite prevalence (Møller et al., 2009). However, this meta-analysis only considered studies of nestling birds and may be fundamentally flawed because it includes observational data as well as experimental data (Borenstein et al., 2009).
The objectives of the present analyses were to review quantitatively experimental studies of wild, free-ranging hosts that measure parasite-induced changes in population-level traits (i.e. measures of fecundity and mortality); then, to evaluate this effect of parasites using life-history traits. Based on those host life-history traits that Møller et al. (2009) found to be significant, the following predictions are made: (1) cavity-nesting species (includes burrowing mammals as well as hollow nesting birds) will experience increased parasite density and intensity and thus more virulent effects than ground or open nesting species (Ewald, 1983); (2) colonial species will experience increased parasite density and intensity and thus more virulent effects than less gregarious species (Ewald, 1983); (3) tropical species will encounter more virulent parasites than temperate species because the absence of seasonality maintains higher parasite abundance (Møller, 1998); and (4) higher virulence will evolve in hosts with shorter life-spans because of the fewer opportunities there are for dispersal to a new host in search of a mate, and as a consequence, the parasites become more virulent (Lehmann, 1993; Nidelet et al., 2009).
2. Materials and methods
2.1. Data collection and inclusion criteria
The studies considered for use in the meta-analysis were obtained from a survey of the primary literature. The initial search was directed using reviews by Møller (1997), Newton (1998), Tompkins and Begon (1999) and Irvine (2006) followed by a comprehensive search of ISI Web of Science and Google Scholar up to and including January 2012. The following search terms and their combinations were used: “parasite∗”, “experiment∗”, “manipulation”, “cost∗”, “effect∗”, “mortality”, “survival”, “fitness”, “host∗”, “life-history”. Older literature (pre-1985) was identified through Literature Cited sections of recent papers and unpublished theses (the same search terms were used in ProQuest Dissertations and Theses, Theses Canada and Trove). Only papers written in English were included. When reference to unpublished work was encountered, attempts were made to solicit raw data from the author(s). A large number of studies were screened using abstracts only (<2000); 89 full-text articles were assessed for eligibility. Of these, 51 were excluded due to a lack of numerical data, lack of sample size and/or variance, untranslatable test statistics, duplication of dataset from a previous paper or reported results not relevant to the selection criteria (e.g. behavioural or physiological/individual responses). Studies were selected if (a) host species were wild (not domesticated), free-ranging (not held in captivity) and the study was conducted under field-conditions (not laboratory conditions); (b) parasite species were experimentally manipulated (increased or decreased); and (c) the parasite was naturally occurring and not introduced, thus avoiding the ‘suicide king’ issue of parasites infecting hosts outside their normal range and becoming more virulent in the process (Dybdahl and Storfer, 2003). Of these, a study was included in the final meta-analyses if it provided (a) the means and standard errors or standard deviations (or any other statistic whereby means and standard errors could be derived) of at least one population-level parameter measuring the cost of parasitism for experimental and control groups, and (b) the sample sizes associated with the means.
2.2. Response variables and calculation of effect sizes
Response variable and effect size data were extracted from the text and tables for all studies except Cheney and Côté (2003), Fitze et al. (2004), Pap et al. (2005), Slomczyński et al. (2006) (additional information requested and received from the authors); and Bize et al. (2004) and Hillegass et al. (2010) (data extracted from graphs using DataThief; Tummers, 2006). Statistics were converted to effect sizes in the form of Hedges’ g (Hedges and Olkin, 1985) in the program Comprehensive Meta-analysis (CMA; Borenstein, 2006). Hedges’ g was chosen as the effect size over the more commonly used Cohen’s d because Hedges’ g pools variance using n − 1 instead of n and thus provides a better estimate for smaller sample sizes (Grissom and Kim, 2005). Studies that reported only F statistics (Møller, 2002; Vandegrift et al., 2008) were not converted to effect sizes due to issues surrounding the overestimation of effect sizes identified by Hullett and Levine (2003) and lack of accurate sample size data in the respective articles. The type of response variable was coded into the data set to enable subgroup analyses. The response variables used were: clutch size (number of eggs in the clutch), percent hatching success (percentage of eggs that hatched from a single clutch), number of young produced (total brood size), percent breeding success (percentage of young produced, fledged or survived during the study period), and survival rate (survival during the study period or between one breeding season and the next).
2.3. Meta-analytic procedures
All meta-analyses were performed in CMA (Borenstein, 2006). A random-effects model was used for all tests because variability was expected in the effects being measured across different species and hosts. Many articles included multiple effect sizes from different measures of the effects of parasitism, so rather than combining all the effect sizes within a study (which may have obfuscated the true effect), in the overall meta-analysis one effect size was chosen at random from each of the forty-three studies (Gurevitch and Hedges, 1999). Separate random-effects meta-analyses were conducted grouped by effect being studied—so any given study might have data in several meta-analyses (sub-analyses) thus maintaining the independence of the data (Gurevitch and Hedges, 1999). One study (Roby et al., 1992) considered the responses of two host species to the same anti-parasitic treatment, so the two hosts were considered as independent studies.
2.4. Heterogeneity and publication bias
Publication bias, or the ‘file drawer problem’, where non-significant results are relegated to the file drawer rather than to the published literature (Rosenberg, 2005), is an ongoing issue affecting meta-analyses, leading to bias via the selective publication of statistically significant results (Hedges and Olkin, 1985). To guard against this issue, publication bias was assessed using three methods: funnel plot (plot of effect size and precision to search for asymmetry), Q-rank correlation (a test for publication bias; Begg and Mazumdar, 1994), and trim-and-fill (Duval and Tweedie, 2000). Heterogeneity indicates the presence of effect-modifiers, and the Q-test for heterogeneity was calculated for the overall meta-analysis. However, because the meta-analysis spans many classes of organism, both as hosts and as parasites, heterogeneity is expected to be high.
2.5. Meta-regression
Meta-regression analyses, a tool used to examine the impact of moderator variable on effect sizes, were performed to assess the degree to which certain life-history traits influence the virulent effects of parasites. These life-history traits (living in cavities/hollows, coloniality, latitude, and host life-span) have been identified as possible predictors to parasite virulence (Møller et al., 2009). Cavity living was coded as 0 for species that lived in the open and 1 for species that lived or bred in tree hollows or underground. Coloniality was coded by maximum recorded colony size: 0 for solitary, 1 for 2–10 pairs, 2 for 11–100 pairs, 3 for 101–1000 pairs and 4 for 1001+ pairs. Latitude was entered using information provided in the source article. Host life-span was coded as maximum recorded (from a wild individual where possible; Carey and Judge, 2002) and average (age an individual which reaches breeding age can be expected to live; Robinson, 2005); any gaps in these two databases were filled using the Encyclopedia of Life.
3. Results
The comprehensive literature search yielded 38 studies that reported the required statistics (or the required statistic was supplied by the author) and so were used in the meta-analysis (Table 1). These studies comprised 31 on avian hosts, 6 on mammalian hosts and 1 on a fish host. There were no studies of reptiles or amphibians that fitted the parameters of this meta-analysis.
Table 1.
Studies used in the meta-analysis investigating using parasite load manipulations the effect of parasites on their wild, free-ranging hosts. Hosts are listed in taxonomic order using common name and parasites are listed by common name. Data for the meta-regression are coded in the following order: living in cavities/hollows (0 = open; 1 = cavity/hollow), coloniality (0 = solitary; 1 = 2–10 pairs; 2 = 11–100 pairs, 3 = 101–1000 pairs; 4 = 1001+ pairs), latitude (in cases where the same species is used from different locations, just the latitude is recorded), and host life-span (maximum recorded; average).
| Host | Parasite | Response variable | Source paper | Meta-regression |
|---|---|---|---|---|
| Piscine Perciformes | ||||
| Longfin Damselfish | Isopods | Clutch size | Cheney and Côté (2003) | 0, 0,13.10, 6, 6 |
| Avian Galliformes | ||||
| Red Grouse | Nematode | Clutch size, hatching success, # young produced | Hudson (1986) | 0, 0, 53.95, 8, 3 |
| Nematodes | # Young produced | Redpath et al. (2006) | ||
| Columbiformes | ||||
| Rock Dove | Lice | Hatching success | Clayton et al. (1999) | 0, 3, 41.15, 35, 6 |
| Ciconiiformes | ||||
| Cattle Egret | Ticks | # Young produced | McKilligan (1996) | 0, 4, 27.55, 17, 8 |
| Pelicaniformes | ||||
| Eastern Brown Pelican | Ticks | Hatching success | Norcross and Bolen (2002) | 0, 3, 33.56, 27, 25 |
| Charadriiformes | ||||
| Eurasian Oystercatcher | Nematodes, trematodes, cestodes | Clutch size, hatching success, # young prod., % succ. | Van Oers et al. (2002) | 0, 0, 53.29, 43, 12 |
| Crested Tern | Lice, ticks | # Young produced | Watson unpub. Data | 0, 4, 38.31, 32, 15 |
| Apodiformes | ||||
| Alpine Swift | Louse-flies | # Young Produced | Bize et al. (2004) | 1, 3, 47.12, 26, 6 |
| Passeriformes (Hirundinidae) | ||||
| Barn Swallow | Mites | Clutch size, hatching success, # young produced | Møller (1990) | 1, 2, 57.12, 10, 6 |
| Mites | Clutch size | Pap et al. (2005) | ||
| Cliff Swallow | Bugs | # Young Produced | Brown and Brown (1986) | 1, 4, 41.13, 11, 5 |
| Bugs, ticks | % Successful | Chapman and George (1991) | 33.20 | |
| Bugs, fleas, lice | Survival rate | Brown et al. (1995) | ||
| Bugs | Survival rate | Brown and Brown (2004) | ||
| Tree Swallow | Blow fly | % Successful | Roby et al. (1992) | 1, 2, 42.59, 12, 3 |
| Fleas | Clutch size, hatching success, # young prod., % succ. | Rendell and Verbeek (1996) | ||
| House Martin | Bugs | Hatching success, % successful, # young produced | de Lope and Møller (1993) | 1, 3, 38.50, 15, 2 |
| Bugs, malaria | Clutch size, hatching success, # young prod., % Succ. | Marzal et al. (2005) | ||
| Purple Martin | Mites | Hatching success, # young produced, % successful | Moss et al. (1966) | 1, 2, 38.58, 13, 8 |
| Sand Martin | Ticks | # Young produced | Szép and Møller (1999) | 1, 3, 48.08, 10, 2 |
| Passeriformes (Turdidae) | ||||
| Eastern Bluebird | Blow fly | % Successful | Roby et al. (1992) | 1, 0, 42.59, 10, 6 |
| Passeriformes (Sturnidae) | ||||
| European Starling | Mites | Hatching success, % successful | Fauth et al. (1991) | 1, 0, 39.45, 15, 5 |
| Passeriformes (Paridae) | ||||
| Blue Tit | Fleas | Clutch size, # young produced | Richner and Tripet (1999) | 1, 0, 46.31, 15, 3 |
| Haematazoa | % Successful | Merino et al. (2000) | 40.48 | |
| Ticks, fleas, blow fly | Clutch size, # young produced | Bouslama et al. (2002) | 36.42 | |
| Haematazoa | Clutch size, hatching success, % successful | Tomás et al. (2007)a | 40.53 | |
| Fleas, blow flies, mites | % Successful | Slomczyński et al. (2006) | 51.47 | |
| Haematazoa | Survival rate | Martínez-de la Puente et al. (2010) | 40.53 | |
| Great Tit | Fleas | # Young produced | Richner et al. (1993) | 1, 0, 46.31, 15, 3 |
| Fleas | Clutch size, # young produced | Opplinger et al. (1994) | 46.31 | |
| Fleas | Clutch size | Fitze et al. (2004) | 46.54 | |
| Mammalian Lagomorpha | ||||
| Snowshoe Hare | Nematodes | # Young produced, survival rate | Bloomer et al. (1995) | 0, 0, 44.18, 5, 1 |
| Mountain Hare | Nematodes | Survival rate | Newey and Thirgood (2004) | 0, 2, 57.00, 9, 4 |
| Rodentia (Cricetidae) | ||||
| Townsend’s Vole | Bot fly, mites, nematodes | Survival rate | Steen et al. (2002) | 1, 0, 49.04, 1, 1 |
| Rodentia (Sciuridae) | ||||
| Cape Ground Squirrel | Ticks, lice, fleas, worms | # Young produced | Hillegass et al. (2010) | 1, 2, 27.35, 6, 4 |
| Colum. Ground Squirrel | Fleas | # Young produced | Neuhaus (2003) | 1, 2, 50.00, 11, 3 |
| Cetartiodactyla | ||||
| Bighorn Sheep | Nematodes | Survival rate | Schmidt et al. (1979) | 0, 2, 37.34, 24, 6 |
3.1. Overall effect and publication bias
The data set were first analysed as a whole (termed combined in Table 1). In order to avoid replication for this combined analysis, one sample was randomly chosen from each study. A forest plot of the effect sizes for all studies showed that twenty-seven reported negative effects of parasites, and eleven showed positive or nil effects (Fig. 1). The mean effects size across all studies was 0.489 (95% CI, 0.220–0.759, n = 38) and was statistically significant from a null effect size (Z = 3.56, p = 0.0004). Additionally, to determine what impact the random sampling might have had on the power of the combined analysis, a meta-analysis was performed on the entire data set resulting in a mean effect size of 0.47 (95% CI, 0.282–0.658, n = 60).
Fig. 1.
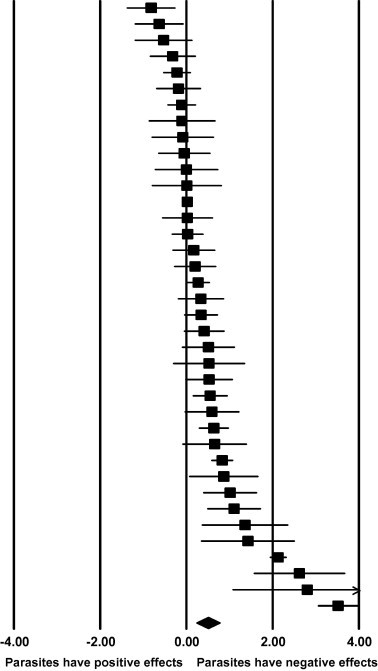
Forest plot of effect sizes (rectangles) and confidence intervals (bars) for each study and the effect averaged across all studies (diamond).
A funnel plot suggested some publication bias (Fig. 2), however, a rank correlation test between precision and effect size (Begg and Mazumdar, 1994) was not statistically significant (Kendall’s τ = 0.105, P = 0.176; one-tailed, with continuity correction). Additionally, the trim-and-fill analysis of the random effects model imputed no missing studies. Despite some evidence to suggest that there was a publication bias, there is much support for the negative effects of parasites at the population level.
Fig. 2.
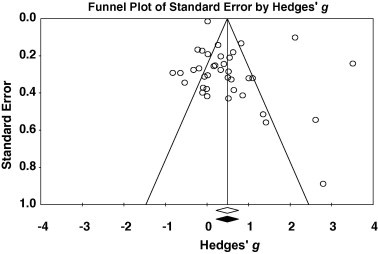
Funnel plot of effect size (Hedges’ g) by standard error (SE). The white circles represent studies included in the meta-analysis. The black circles represent missing imputed studies. The white diamond represents overall effect size as calculated in the meta-analysis, and the black diamond represents the corrected effect size.
3.2. Subgroup analyses
As expected, there was significant heterogeneity in effect sizes across studies (Q = 749.54, df = 37, p < 0.0001), thus, subgroup analyses on response variables were analysed, both to examine possible variables influencing the results and to examine the data from a biologically relevant point of view. The complete data set (not the randomly selected subset) were separated into the response variable measured—clutch size (n = 12), percent hatching success (n = 11), number of young produced (n = 20), percent breeding success (n = 12), and survival rate (n = 7). A meta-analysis was performed on each group (Table 2) and revealed significant negative effects of parasites on clutch size, hatching success, and young produced, but not overall breeding success or survival rate.
Table 2.
The influence of response variable measured on population-level host response to parasites. Data points represent mean effect sizes (Hedges g) and their 95% confidence intervals (CI), as estimated in the meta-analyses. Combined refers to an overall averaged effect size from randomly taking one response from each study. Positive values indicate that the parasite had a negative effect on the host, while negative numbers indicate that the parasite had a neutral to positive effect on the host. Where 95% CI do not include zero, the effect can be considered to be statistically significant.
| Response variable | Mean ± SE | 95% CI | Z-Value | p-Value | Number of studies |
|---|---|---|---|---|---|
| Clutch size | 0.365 ± 0.14 | 0.094–0.636 | 2.636 | 0.008 | 12 |
| Hatching success | 0.310 ± 0.11 | 0.097–0.515 | 2.873 | 0.004 | 11 |
| Young produced | 0.596 ± 0.16 | 0.286–0.906 | 3.772 | 0.0002 | 20 |
| % Breeding success | 0.219 ± 0.34 | −0.447 to 0.886 | 0.644 | 0.520 | 12 |
| Survival rate | 0.672 ± 0.35 | −0.007 to 1.351 | 1.939 | 0.053 | 7 |
| Combined | 0.489 ± 0.14 | 0.220–0.759 | 3.56 | 0.0004 | 38 |
3.3. Meta-regression of life history traits
The effect sizes from the complete data set (n = 60; not the randomly sampled subset used in the overall meta-analysis) were regressed against five moderator variables—cavities/hollow living, coloniality, latitude (chosen from Møller et al. (2009) paper on variables that significantly impact chick survival), maximum host life-span, and average host life-span (chosen as a more precise measure of intrinsic host mortality than survival rate used by Møller et al., 2009). The proportion of variance explained by all these moderator variables was nonsignificant except for average age (significant at p = 0.05) (Table 3; Fig. 3).
Table 3.
Meta-regression (random-effects model) results for moderator variables as a function of the effects of parasites. The influence of response variable measured on population-level host response to parasites. Point estimates are for intercepts and the Z-test is used to test that slope is not zero.
| Moderator variable | Point estimate ± SE | 95% CI | Z-Value | p-Value |
|---|---|---|---|---|
| Cavity/hollow living | 0.0005 ± 0.01 | −0.029–0.030 | 0.034 | 0.97 |
| Coloniality | 0.008 ± 0.02 | −0.034–0.050 | 0.36 | 0.72 |
| Latitude | −0.01 ± 0.01 | −0.038–0.008 | −1.30 | 0.19 |
| Maximum lifespan | −0.01 ± 0.01 | −0.034–0.005 | −1.44 | 0.15 |
| Average lifespan | −0.05 ± 0.02 | −0.094–0.00064 | −1.93 | 0.05 |
Fig. 3.
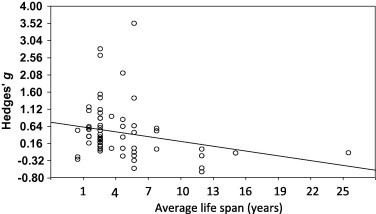
A meta-regression (random effects model) of effect size against host average lifespan using the complete dataset (n = 60). The slope of this regression is significant (p = 0.05).
4. Discussion
A meta-analysis of 38 experimental studies of the costs of parasites to population-level measures of natural, free-ranging hosts revealed an overall effect size, g, of 0.49. These parasites, therefore, produced a moderate (as defined by Cohen, 1988) negative effect. This effect size can be interpreted as an average difference of 0.49 standard deviations between parasitised and non-parasitised individuals. This effect is as strong as the reported effect sizes of predators on populations. Côté and Sutherland (1997) quantified, using a meta-analysis, the effect size of predation upon population size in birds. The summary effect size was either 0.34 or 0.95 depending on the way the population size was measured (either post-breeding or overall population size). Gurevitch et al. (2000) examined the combined effects of predation and competition on population sizes of anurans in field experiments. In this case, the summary effect size is obfuscated by interactions between predation and competition, but the authors state that “the average overall effects of predator exclusion were very large” and the average effect of competitor removal in the presence of predators on survival was −0.35 (negatively effecting survival). Given the summary effect size reported in this study, the indication is that parasites may be at least as important as predation (or predation combined with competition) in their effects on populations. It is important to include parasites in future research that records demographic information. By incorporating parasite removal experiments into predation- or competition-based experiments, the interactions between three demographic forces will yield a broader understanding of host–parasite interactions (e.g. Holt and Roy, 2007).
Meta-analyses of the response variables indicated that clutch size, hatching success, and number of young produced were all significantly reduced in parasitised individuals. The only response variables that was not significantly affected by parasites was breeding success (defined variously as percentage of young produced, fledged or survived during the study period) and survival (although sample size was very low for survival rate, p = 0.053, thus more studies may indicate that survival is indeed significantly reduced by parasites). Further analyses using meta-regression to determine the key life-history traits that may be driving the observed population-level effects of parasites revealed that the most likely key driver of effects of a parasite on its host was host life-span. Other variables previously suggested (Møller et al., 2009) as determinants of virulence—cavity/hollow living, coloniality or latitude were not significant.
There are strongly suggestive mathematical models (Anderson, 1978; Anderson and May, 1978; May and Anderson, 1978) and some empirical evidence (Gregory, 1991; Hudson et al., 1998) that parasites can regulate host populations and cycles. The large effect size revealed in this meta-analysis supports the idea that parasites are a major force in population regulation in wild, free-ranging vertebrate host populations. Support for the idea of the importance of parasites has far-reaching implications in both ecological and conservation-based science. If parasites are such strong regulators, why are some species seemingly untouched by the effects of parasites despite large parasite loads? Are parasites simply another form of predation? Should parasites be removed in populations needing conservation regulation, or, conversely, added to invasive populations? Unfortunately, before drawing a longbow based around this meta-analysis, it is important to examine the taxonomy of the host species that went into the model. While this meta-analysis does include studies from twenty-four different species, they are mainly clustered within one order of one class of host study organism. The taxonomic bias of host species studied must temper any conclusions—these results may only be applicable to the hosts used in the meta-analysis. It may be that the effects of parasites as measured here are inflated compared to other sorts of hosts and the actual overall effects of natural parasites are much less.
Given these caveats, the information provided from this meta-analysis still lends itself to hypothesis generation and testing around the life-history traits that drive the evolution of a parasite to cause damage to its host. The results of the meta-regression, which refuted all previous predictions regarding the determinants of parasite virulence, suggest that intrinsic host mortality (lifespan) is the key to understanding why and how parasites evolve to either harm or be benign to their host. Although the significance of this result is not high, it may suggest why observed parasite virulence varies so widely between host species. The evolutionary idea that background host mortality may explain an increase in virulence has been demonstrated in the laboratory (Nidelet et al., 2009). Additionally, longer host lifespan have been shown to evolve under high condition dependent morality (Williams and Troy, 2003; Dowling, 2012). Combining these two ideas with the results of this meta-analysis and meta-regression suggests that we should expect long-lived hosts to have non-virulent parasites except when the host’s condition is poor. Moreover, short-lived hosts should show increased responses to parasites regardless of their own condition. Therefore, in order to increase our understanding of the effects of parasites on wild populations, focused studies on long-lived hosts are vital. Especially, where long-lived and short-lived examples can be found within the same taxonomic group.
The surprising lack of support for the hypotheses that parasites should be more virulent with cavity/hollow living, higher numbers of individuals living or breeding together, and in tropical latitudes is illustrative of the conundrum of mixing the results of observational studies with experimental ones. Intuitively, one expects that species living or breeding in hollows, underground or in a colony would experience greater abundance and prevalence of parasites due to repeated use of the living area. However, the number of parasites on an individual host does not necessarily indicate an increased effect of that parasite on the host. Additionally, when one considers observational data, the researcher is asking if parasitised individuals are more affected by parasites than those individuals who are naturally uninfected, while an experimental study seeks to understand if parasites have more effects regardless of the chance or reason for having become infected in the first place.
In conclusion, this study provides a quantitative test of the effects of parasites to their wild, free-ranging vertebrate hosts. The results suggest that parasites are indeed drivers of population-level life-history traits, as has been advocated for over thirty years. However, the results are heavily taxonomically biased, and therefore, may only be applicable to a limited number of species and scenarios. Interrogation of the data using meta-regression revealed that the one life-history trait that appears to drive the capacity of a parasite to negatively impact its host is intrinsic host lifespan. However, it is difficult to separate the relative importance of these life-history traits in shaping parasite virulence, and more data are needed to understand these phenomena. An examination of the effects of parasites on long-lived hosts is warranted to understand fully the magnitude and extent of the effects of parasites in natural situations.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Albon S.D., Stien A., Irvine R.J., Langvatn R., Ropstad E., Halvorsen O. The role of parasites in the dynamics of a Reindeer population. Proceedings of the Royal Society of London B. 2002;269:1625–1632. doi: 10.1098/rspb.2002.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M. The regulation of host population growth by parasitic species. Parasitology. 1978;76:119–157. doi: 10.1017/s0031182000047739. [DOI] [PubMed] [Google Scholar]
- Anderson R.M., May R.M. Regulation and stability of host–parasite population interactions: I. Regulatory processes. Journal of Animal Ecology. 1978;47:219–247. [Google Scholar]
- Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Bize P., Roulin A., Tella J.L., Bersier L.-F., Richner H. Additive effects of ectoparasites over reproductive attempts in the long-lived Alpine Swift. Journal of Animal Ecology. 2004;73:1080–1088. [Google Scholar]
- Bize P., Jeanneret C., Klopfenstein A., Roulin A. What makes a host profitable? Parasites balance host nutritive resources against immunity. The American Naturalist. 2008;171:107–118. doi: 10.1086/523943. [DOI] [PubMed] [Google Scholar]
- Bloomer S.E.M., Willebrand T., Keith I.M., Keith L.B. Impact of helminth parasitism on a snowshoe hare population in central Wisconsin: a field experiment. Canadian Journal of Zoology. 1995;73:1891–1898. [Google Scholar]
- Borenstein, M., Hedges, L.V., Higgins, J.P.T., Rothstein, H.R., 2006. Comprehensive Meta-Analysis (Version 2.2.064) [Computer software]. Englewood, NJ: Biostat.
- Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. John Wiley & Sons, Ltd.; West Sussex, UK: 2009. Introduction to Meta-Analysis. [Google Scholar]
- Bouslama Z., Lambrechts M.M., Ziane N., Djenidi R., Chabi Y. The effect of nest-ectoparasites on parental provisioning in a north-African population of the Blue Tit Parus caeruleus. Ibis. 2002;144:E73–E78. [Google Scholar]
- Brown C.R., Brown M.B. Ectoparasitism as a cost of coloniality in Cliff Swallows (Hirundo pyrrhonota) Ecology. 1986;67:1206–1218. [Google Scholar]
- Brown C.R., Brown M.B., Rannala B. Ectoparasites reduce long-term survival of their avian host. Proceedings of the Royal Society of London B. 1995;262:313–319. [Google Scholar]
- Brown C.R., Brown M.B. Group size and ectoparasitism affect daily survival probability in a colonial bird. Behavioral Ecology and Sociobiology. 2004;56:498–511. [Google Scholar]
- Carey J.R., Judge D.S. Odense University Press; Odense: 2002. Longevity Records: Life Spans of Mammals, Birds, Amphibians, Reptiles, and Fish. [Google Scholar]
- Casadevall A., Pirofski L.-A. Host–pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infection and Immunity. 1999;67:3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B.R., George J.E. The effects of ectoparasites on Cliff Swallow growth and survival. In: Loye J.E., Zuk M., editors. Bird–Parasite Interactions: Ecology, Evolution and Behaviour. Oxford University Press; Oxford: 1991. [Google Scholar]
- Cheney K.L., Côté I.M. The ultimate effect of being cleaned: does ectoparasite removal have reproductive consequences for Damselfish clients? Behavioral Ecology. 2003;14:892–896. [Google Scholar]
- Clayton D.H., Lee P.L.M., Tompkins D.M., Brodie E.D., III Reciprocal natural selection on host–parasite phenotypes. The American Naturalist. 1999;154:261–270. doi: 10.1086/303237. [DOI] [PubMed] [Google Scholar]
- Cohen J. second ed. Academic Press; New York: 1988. Statistical Power Analysis for the Behavioural Sciences. [Google Scholar]
- Combes, C., 2005. The Art of Being a Parasite (trans. by D Simberloff). University of Chicago Press, Chicago.
- Côté I.M., Sutherland W.J. The effectiveness of removing predators to protect bird populations. Conservation Biology. 1997;11:395–405. [Google Scholar]
- de Lope F., Møller A.P. Effects of ectoparasites on reproduction of their swallow hosts: a cost of being multi-brooded. Oikos. 1993;67:557–562. [Google Scholar]
- Diamond J.M. Laboratory, field and natural experiments. Nature. 1983;304:586–587. [Google Scholar]
- Dowling D.K. Ageing: evolution of life span revisited. Current Biology. 2012;22:R947–R949. doi: 10.1016/j.cub.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Duval S.J., Tweedie R.L. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Dybdahl M.F., Storfer A. Parasite local adaptation: Red Queen versus Suicide King. Trends in Ecology and Evolution. 2003;18:523–530. [Google Scholar]
- Encyclopedia of Life. Available from <http://www.eol.org>.
- Ewald P.W. Host–parasite relations, vectors, and the evolution of disease severity. Annual Review of Ecology, Evolution, and Systematics. 1983;14:465–485. [Google Scholar]
- Fauth P.T., Krementz D.G., Hines J.E. Ectoparasitism and the role of green nesting material in the European Starling. Oecologia. 1991;88:22–29. doi: 10.1007/BF00328399. [DOI] [PubMed] [Google Scholar]
- Fitze P.S., Tschirren B., Richner H. Life history and fitness consequences of ectoparasites. Journal of Animal Ecology. 2004;73:216–226. [Google Scholar]
- Gregory R.D. Parasite epidemiology and host population growth: Heligmosomoides polygyrus (Nematoda) in enclosed Wood Mouse populations. Journal of Animal Ecology. 1991;60:805–821. [Google Scholar]
- Grissom R.J., Kim J.J. Taylor and Francis; New York: 2005. Effect Sizes for Research. [Google Scholar]
- Gulland F.M.D. The role of nematode parasites in Soay Sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- Gurevitch J., Hedges L.V. Statistical issues in conducting ecological meta-analyses. Ecology. 1999;80:1142–1149. [Google Scholar]
- Gurevitch J., Morrison J.A., Hedges L.V. The interaction between competition and predation: a meta-analysis of field experiments. The American Naturalist. 2000;155:435–453. doi: 10.1086/303337. [DOI] [PubMed] [Google Scholar]
- Hedges L.V., Olkin I. Academic Press; London: 1985. Statistical Methods for Meta-Analysis. [Google Scholar]
- Hillegass M.A., Waterman J.A., Roth J.D. Parasite removal increases reproductive success in a social African Ground Squirrel. Behavioral Ecology. 2010;21:696–700. [Google Scholar]
- Holmes J.C. Evolutionary relationships between parasitic helminths and their hosts. In: Futuyma D.J., Slatkin M., editors. Coevolution. Sinauer Associates, Inc.; Sunderland, MA: 1983. [Google Scholar]
- Holt R.D., Roy M. Predation can increase the prevalence of infectious disease. The American Naturalist. 2007;169:690–699. doi: 10.1086/513188. [DOI] [PubMed] [Google Scholar]
- Hudson P.J. The effect of a parasitic nematode on the breeding production of Red Grouse. Journal of Animal Ecology. 1986;55:85–92. [Google Scholar]
- Hudson P.J., Dobson A.P., Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- Hullett C.R., Levine T.R. The overestimation of effect sizes from F values in meta-analysis: the cause and a solution. Communication Monographs. 2003;70:52–67. [Google Scholar]
- Irvine R.J. Parasites and the dynamics of wild mammal populations. Animal Science. 2006;82:775–781. [Google Scholar]
- Lailvaux S.P., Kasumovic M.M. Defining individual quality over lifetimes and selective contexts. Proceedings of the Royal Society of London B. 2011;211:321–328. doi: 10.1098/rspb.2010.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T. Ectoparasites: direct impact on host fitness. Parasitology Today. 1993;9:8–13. doi: 10.1016/0169-4758(93)90153-7. [DOI] [PubMed] [Google Scholar]
- Martínez-de la Puente J., Merino S., Tomás G., Moreno J., Morales J., Lobato E., García-Fraile S., Belda E.J. The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biology Letters. 2010;6:663–665. doi: 10.1098/rsbl.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzal A., de Lope F., Navarro C., Møller A.P. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia. 2005;142:541–545. doi: 10.1007/s00442-004-1757-2. [DOI] [PubMed] [Google Scholar]
- May R.M., Anderson R.M. Regulation and stability of host–parasite population interactions: II. Destabilizing processes. Journal of Animal Ecology. 1978;47:249–267. [Google Scholar]
- McCallum H. Modelling wildlife–parasite interactions to help plan and interpret field studies. Wildlife Research. 1995;22:21–29. [Google Scholar]
- McKilligan N.G. Field experiments on the effect of ticks on breeding success and chick health of Cattle Egrets. Australian Journal of Ecology. 1996;21:442–449. [Google Scholar]
- Merino S., Moreno J., Sanz J.J., Arriero E. Are avian blood parasites pathogenic in the wild? A medication experiment in Blue Tits (Parus caeruleus) Proceedings of the Royal Society of London B. 2000;267:2507–2510. doi: 10.1098/rspb.2000.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis Y., Olivieri I., Renaud F., Raymond M. Pleiotropic action of parasites: how to be good for the host. Trends in Ecology and Evolution. 1992;7:59–62. doi: 10.1016/0169-5347(92)90108-N. [DOI] [PubMed] [Google Scholar]
- Møller A.P. Effects of parasitism by a haematophagous mite on reproduction in the Barn Swallow. Ecology. 1990;71:2345–2357. [Google Scholar]
- Møller A.P. Parasitism and the evolution of host life history. In: Clayton D.H., Moore J., editors. Host–parasite Evolution: General Principals and Avian Models. Oxford University Press; Oxford: 1997. [Google Scholar]
- Møller A.P. Evidence of larger impact of parasites on hosts in the tropics: investment in immune function within and outside the tropics. Oikos. 1998;82:265–270. [Google Scholar]
- Møller A.P. Temporal change in mite abundance and its effect on Barn Swallow reproduction and sexual selection. Journal of Evolutionary Biology. 2002;15:495–504. [Google Scholar]
- Møller A.P. Parasitism and the regulation of host populations. In: Thomas F., Renaud F., Guégan J.-F., editors. Parasitism and Ecosystems. Oxford University Press; Oxford: 2005. [Google Scholar]
- Møller A.P., Arriero E., Lobato E., Merino S. A meta-analysis of parasite virulence in nestling birds. Biological Reviews. 2009;84:567–588. doi: 10.1111/j.1469-185X.2009.00087.x. [DOI] [PubMed] [Google Scholar]
- Moss, W.W., 1966. The biological and systematic relationships of the Martin Mite, Dermanyssus prognephilus Ewing. Unpublished PhD Thesis. The University of Kansas: Lawrence, Kansas, USA.
- Neuhaus P. Parasite removal and its impact on litter size and body condition in Columbian Ground Squirrels (Spermophilus columbianus) Proceedings of the Royal Society of London B. 2003;270:S213–S215. doi: 10.1098/rsbl.2003.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey S., Thirgood S.J. Parasite-mediated reduction in fecundity of Mountain Hares. Proceedings of the Royal Society of London B. 2004;271:S413–S415. doi: 10.1098/rsbl.2004.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton I. Academic Press; London: 1998. Population Limitation in Birds. [Google Scholar]
- Nidelet T., Koella J.C., Kaltz O. Effects of shortened host life span on the evolution of parasite life history and virulence in a microbial host–parasite system. BMC Evolutionary Biology. 2009;9:65. doi: 10.1186/1471-2148-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross N.L., Bolen E.G. Effectiveness of nest treatments on tick infestations in the Eastern Brown Pelican. Wilson Bulletin. 2002;114:73–78. [Google Scholar]
- Opplinger A., Richner H., Christe P. Effect of an ectoparasite on lay date, nest-site choice, desertion, and hatching success in the Great Tit (Parus major) Behavioral Ecology. 1994;5:130–134. [Google Scholar]
- Pap P.L., Tökölyi J., Szép T. Host–symbiont relationship and abundance of feather mites in relation to age and body condition of the Barn Swallow (Hirundo rustica): an experimental study. Canadian Journal of Zoology. 2005;83:1059–1066. [Google Scholar]
- Poulin R. Are there general laws in parasite ecology? Parasitology. 2007;134:763–776. doi: 10.1017/S0031182006002150. [DOI] [PubMed] [Google Scholar]
- Redpath S.M., Mougeot F., Leckie F.M., Elston D.A., Hudson P.J. Testing the role of parasites in driving the cyclic population dynamics of a gamebird. Ecology Letters. 2006;9:410–418. doi: 10.1111/j.1461-0248.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Renaud F., de Meeüs T. A simple model of host–parasite evolutionary relationships. Parasitism: compromise or conflict? Journal of Theoretical Biology. 1991;152:319–327. doi: 10.1016/s0022-5193(05)80197-3. [DOI] [PubMed] [Google Scholar]
- Rendell W.B., Verbeek N.A.M. Old nest material in nestboxes of Tree Swallows: effects on reproductive success. Condor. 1996;98:142–152. [Google Scholar]
- Richner H., Tripet F. Ectoparasitism and the trade-off between current and future reproduction. Oikos. 1999;86:535–538. [Google Scholar]
- Richner H., Oppliger A., Christe P. Effect of an ectoparasite on reproduction in Great Tits. Journal of Animal Ecology. 1993;62:703–710. [Google Scholar]
- Robinson, R.A., 2005. BirdFacts: profiles of birds occurring in Britain and Ireland (BTO Research Report 407). BTO, Thetford. Available from: <http://www.bto.org/birdfacts>.
- Roby D.D., Brink K.L., Wittmann K. Effects of bird Blow Fly parasitism on Eastern Bluebird and Tree Swallow nestlings. Wilson Bulletin. 1992;104:630–643. [Google Scholar]
- Rosenberg M.S. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59:464–468. [PubMed] [Google Scholar]
- Schmidt R.L., Hibler C.P., Spraker T.R., Rutherford W.H. An evaluation of drug treatment for lungworm in Bighorn Sheep. Journal of Wildlife Management. 1979;43:461–467. [Google Scholar]
- Schmidt-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society of London B. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz A., Ratte H.T. Aquatic ecotoxicology: on the problems of extrapolation from laboratory experiments with individuals and populations to community effects in the field. Comparative Biochemistry and Physiology C: Toxicology and Pharmacology. 1991;100:301–304. [Google Scholar]
- Slomczyński R., Kaliński A., Wawrzyniak J., Bańbura M., Skwarska J., Zieliński P., Bańbura J. Effects of experimental reduction in nest micro-parasite and macro-parasite loads on nesting hemoglobin level in Blue Tits Parus caeruleus. Acta Oecologica. 2006;30:223–227. [Google Scholar]
- Steen H., Taitt M., Krebs C.J. Risk of parasite-induced predation: an experimental field study on Townsend’s Voles (Microtus townsendii) Canadian Journal of Zoology. 2002;80:1286–1292. [Google Scholar]
- Stewart G. Meta-analysis in applied ecology. Biology Letters. 2010;6:78–81. doi: 10.1098/rsbl.2009.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szép T., Møller A.P. Cost of parasitism and host immune defence in the Sand Martin Riparia riparia: a role for parent-offspring conflict? Oecologia. 1999;119:9–15. doi: 10.1007/s004420050755. [DOI] [PubMed] [Google Scholar]
- Tomás G., Merino S., Moreno J., Morales J. Consequences of nest reuse for parasite burden and female health and condition in Blue Tits, Cyanistes caeruleus. Animal Behaviour. 2007;73:805–814. [Google Scholar]
- Tompkins D.M., Begon M. Parasites can regulate wildlife populations. Parasitology Today. 1999;15:311–313. doi: 10.1016/s0169-4758(99)01484-2. [DOI] [PubMed] [Google Scholar]
- Tummers, B., 2006. DataThief III version 1.6. Available from: <http://datathief.org/>.
- Vandegrift K.J., Raffel T.R., Hudson P.J. Parasites prevent summer breeding in white-footed mice, Peromyscus leucopus. Ecology. 2008;89:2251–2258. doi: 10.1890/07-1935.1. [DOI] [PubMed] [Google Scholar]
- Van Oers K., Heg D., Quenec’hdu S.L.D. Anthelminthic treatment negatively affects chick survival in the Eurasian Oystercatcher Haematopus ostralegus. Ibis. 2002;144:509–517. [Google Scholar]
- Williams P.D., Troy D. Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution. 2003;57:1478–1488. doi: 10.1111/j.0014-3820.2003.tb00356.x. [DOI] [PubMed] [Google Scholar]


