Graphical abstract
Highlights
► This is the first study on the taeniid species infecting the Portuguese population of Iberian wolf. ► Taeniid species detected are strongly related to the feeding habits of this top carnivore. ► First report of Taenia polyacantha and Echinococcus intermedius in the Iberian wolf. ► A genuine wild cycle for E. intermedius might persist between wolves and wild boars in this region.
Keywords: Echinococcus intermedius, Taenia spp., Iberian wolf, Portugal
Abstract
Taeniid species represent relevant pathogens in human and animals, circulating between carnivorous definitive hosts and a variety of mammalian intermediate hosts. In Portugal, however, little is known about their occurrence and life cycles, especially in wild hosts. An epidemiological survey was conducted to clarify the role of the Iberian wolf as a definitive host for taeniid species, including Echinococcus spp. Wolf fecal samples (n = 68) were collected from two regions in Northern Portugal. Taeniid eggs were isolated through a sieving-flotation technique, and species identification was performed using multiplex-PCR followed by sequencing of the amplicons. Taenia hydatigena (in 11.8% of the samples), Taenia serialis (5.9%), Taenia pisiformis (2.9%), Taenia polyacantha (1.5%) and Echinococcus intermedius (Echinococcus granulosus ‘pig strain’, G7) (1.5%) were detected. This is the first study to characterize the taeniid species infecting the Portuguese Iberian wolf, with the first records of T. polyacantha and E. intermedius in this species in the Iberian Peninsula. Iberian wolves can be regarded as relevant hosts for the maintenance of the wild and synanthropic cycles of taeniids in Portugal.
1. Introduction
The Iberian wolf (Canis lupus signatus, Cabrera 1907) is an endangered carnivore subspecies that inhabits the Northern Iberian Peninsula. In Portugal, where a population of 200–400 individuals is estimated, the habitats of this top predator overlap rural human communities, where wolves have access to domestic ungulates, their main preys (Álvares et al., 2000; Pimenta et al., 2005). On such occasions with a close contact between wolves, domestic animals and humans, transmission of pathogens, especially those whose life cycle is based on predator-prey interactions, might occur. Taeniid species (Cestoda: Taeniidae) are a remarkable example of this One Health perspective. Several taeniid species have been described in animals and humans in Portugal, including the zoonotic Echinococcus granulosus sensu lato, which, although in a lesser degree than in the past, is still responsible for human morbidity as well as ungulate offal rejection, mainly in sheep (Beato et al., 2010; David de Morais, 2010). Two E. granulosus genotypes have so far been described in Portugal, namely E. granulosus sensu stricto (G1) in cattle and sheep, and E. intermedius in domestic pigs (formerly E. granulosus G7) (Castro et al., 2005; Thompson, 2008; Beato et al., 2010). Despite their medical and veterinary relevance, these parasites have been poorly investigated in the last decades, and information on wild definitive hosts, possible sources of infection for both humans and livestock, are lacking.
The aim of this study was to evaluate for the first time the involvement of the Portuguese population of Iberian wolf as definitive host of taeniids.
2. Materials and methods
Sixty-eight (n = 68) Iberian wolf fecal samples were collected between October 2008 and January 2009 and stored at 4 °C. In order to specifically collect wolf samples, transects were chosen at the areas most intensively used by wolves as established in the frame of the national wolf census performed in 2002/2003 (Pimenta et al., 2005). Identification of field samples was done under supervision of experienced field biologists, taking into account morphology, content, odor, as well as additional factors (e.g. absence of stray dogs, reports of wolf attacks to domestic animals).
The majority of the samples (n = 57) were collected from an area North of the River Douro, where the main Portuguese wolf population occurs (Fig. 1a.1). Samples were collected from the territories of eight known wolf packs (Fig. 1b). A second group of samples (n = 11) was collected from an area South of the river Douro, inhabited by a smaller isolated subpopulation (Pimenta et al., 2005) (Fig. 1a.2). These two study areas are mainly mountainous, with some dispersed rural, aged human populations strongly dependent on agriculture and farming.
Fig. 1.
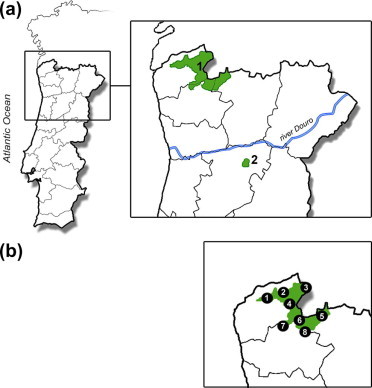
Sampling areas in the North of Portugal. (a) In the northern area (1) 57 fecal samples were collected and in Leomilde, the southern area (2), 11 samples; (b) Approximate location of samples in the northern area, named according to the corresponding wolf pack territories [(1) Boulhosa (n = 1), (2) Vez (n = 20), (3) Castro Laboreiro (n = 1), (4) Soajo (n = 7), (5) Pitões (n = 4), (6) Amarela (n = 10), (7) Vila Verde (n = 1) and (8) Gerês (n = 13)].
Taeniid eggs were isolated through a combined method of flotation in zinc chloride solution (density 1.45 g/mL) and sieving (Mathis et al., 1996) and identified under an inverted microscope. To increase the sensitivity, two aliquots of 2 g per sample were analyzed.
The material was not deep-frozen at −80 °C before processing since the samples were also used for another study about Toxocara, whose eggs are destroyed or deformed with this step (data not shown). However, all other safety procedures (Eckert et al., 2001) were strictly followed.
DNA extraction was carried out as described by Štefanić et al. (2004). Species identification of taeniid egg-positive samples was done by a multipex-PCR, according to Trachsel et al. (2007), using a Qiagen multiplex PCR kit (Qiagen, Hilden, Germany). Furthermore, Taenia spp. and E. granulosus sensu lato amplicons were identified to species level through direct sequencing, after purification using the MinElute PCR purification kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Sequencing was performed by Synergene Biotech GmbH, Biotech Center Zurich, Switzerland (http://www.synergene-biotech.com) with the primers Cest5seq and Cest4 for Taenia spp. and E. granulosus s.l., respectively (Trachsel et al., 2007). Sequencing results were then compared with entries in the GenBank nucleotide database, using BLAST search (http://www.blast.ncbi.nlm.nih.gov).
Since genotyping to determine individual wolves was not performed for these fecal samples, some of them may have originated from the same animal and, thus, the term occurrence rather than prevalence is used to describe the percentage of positives.
To estimate the occurrence of taeniid eggs as well as its confidence interval (95%), the free software Quantitative Parasitology 3.0 was used [Reiczigel and Rózsa (2005)].
3. Results and discussion
The overall occurrence for taeniid eggs was 23.5%, which is in agreement with previous results based on coprology and necropsy of wolves in nearby regions (Torres et al., 2000; Segovia et al., 2001; Silva, 2010). Sequencing revealed E. intermedius and four Taenia species (Table 1).
Table 1.
Absolute frequency and occurrence (%) of taeniid eggs isolated from 68 Iberian wolf fecal samples and genetically identified.
| Taeniid species | Frequency/occurrence (%) [Confidence interval 95%] |
|---|---|
| Taeniid eggs | 16 23.5 [14.5–35.2] |
| E. intermedius (G7) | 1 1.5 [0.8–7.8] |
| Taenia hydatigena | 8 11.8 [5.5–21.9] |
| Taenia serialis | 4 5.9 [2.0–14.5] |
| Taenia pisiformis | 2 2.9 [0.5–10.1] |
| Taenia polyacantha | 1 1.5 [0.1–7.8] |
The most important result of this study was the finding of E. intermedius for the first time in the Iberian wolf and, as far as we know, also for the first time in wolves in Europe. Although the sequence obtained was short (75 bp), GenBank analysis revealed 100% identity only with E. intermedius (E. granulosus G6/G7 genotypes), namely a G7 isolate from a pig in Slovakia (Genbank accession number: AY462128). E. intermedius is known to occur in domestic pigs in the Northern part of the country (Castro et al., 2005) and also in wild boars and goats in Spain (Mwambete et al., 2004). Highest fertility rates are found in hydatid cysts from wild and domestic swine species (Mwambete et al., 2004), which might be the crucial intermediate hosts for this genotype in the Iberian Peninsula. The positive sample for E. intermedius originated from the subpopulation south of the river Douro (Fig. 1area 2) where wild boars are more common and represent the second most frequently predated species by wolves (Carreira and Petrucci-Fonseca, 2000). Therefore, it can be concluded that probably a genuine wild animal cycle persists, independent of human activity. However, as wolves in this area are often necrophagous and feed on domestic animal carcasses from illegal dumping sites (Pimenta et al., 2005), a synanthropic cycle between wolves and domestic pigs cannot be excluded.
Few wild animal cycles are described for E. granulosus sensu lato in Europe, e.g. wolves and cervids in Northern Scandinavia (Hirvelä-Koski et al., 2003) and nearby Baltic countries (Moks et al., 2006). Other examples throughout the world include dingoes and wild macropodids (kangaroos and wallabies) (Jenkins, 2006) in Australia or wolves and cervids in North America (Jenkins et al., 2011). With the exception of the wild animal cycle in Australia, which was created by human introduction, these life cycles are believed to represent ancestral cycles.
The results of this study are in agreement with the current knowledge about the epidemiology of echinococcosis in Portugal (Castro et al., 2005; Beato et al., 2010; David de Morais, 2010). Due to extensive raising of sheep, lack of hygiene education and close contact between dogs, sheep and humans, there is a predominance of the E. granulosus ‘sheep strain’ (G1) in Southern Portugal. In the North, where pigs are a more important resource for human communities, the E. granulosus ‘pig strain’ (G7) seems more prevalent. Up to date, no genetic studies are available addressing the E. granulosus genotype infecting humans in Portugal. The few analyzed isolates in Spain all belonged to E. granulosus sensu stricto (G1) (Mwambete et al., 2004); however, human cases with E. intermedius (G7) are common in other European countries (Schneider et al., 2010). Further studies in this area would be useful in clarifying the importance of E. intermedius as a zoonotic agent.
The most common taeniid was T. hydatigena with an overall occurrence of 11.8%. It was found at four locations (Gerês, Vez, Amarela and Leomilde) both in the northern and southern areas revealing a wide distribution through the wolf populations. This Taenia species is often considered a core species in the helminth fauna of wolves in Europe (Craig and Craig, 2005), whose main preys are ungulates, well-known intermediate hosts of this parasite. Most likely, there is a synanthropic life cycle involving wolves and domestic animals in the North of Portugal, but also a wild one between wolves and wild ungulates (cervids and wild boar) might exist. Shepherd dogs area it probably also an important definitive host and a source of infection for the domestic ungulates.
Both Taenia serialis and Taenia pisiformis use as intermediate hosts lagomorphs and rodents, which are of minor importance in the wolves’ diet in this region (Álvares et al., 2000; Carreira and Petrucci-Fonseca, 2000). Their occurrence was low and localized. T. pisiformis was found only in the southern area while T. serialis in Pitões and Amarela, two contiguous areas. Likewise, Taenia polyacantha, whose intermediate hosts are rodents, was for the first time recorded in Iberian wolves, but with a low occurrence (only one positive sample from Vez). In Eastern Europe, it is a known parasite of wolves (Craig and Craig, 2005) and although no information exists regarding its intermediate hosts in Portugal, T. polyacantha had already been found in foxes in nearby regions (Carvalho-Varela and Marcos, 1993). Given their respective intermediate hosts, all these three taeniid species mainly circulate in a wild cycle. Attention must be paid since high infection rates can be detected in important game resources. For instance, frequent findings of T. pisiformis cysticerci in hunted wild rabbits and hares (Madeira de Carvalho and Valverde, unpublished) entails significant economic losses.
A final remark should be made concerning the importance of the methodological approach used in this study. Wildlife specimens, especially the ones from protected species, are frequently not available for necropsy examination which represents the gold standard for most intestinal parasites. Therefore, identifying parasite infections through the isolation of their eggs in feces is a valuable tool. As taeniid eggs cannot be differentiated by morphology, a sieving-flotation technique followed by multiplex-PCR and sequencing was applied for species or genotype identification (Deplazes et al., 2003; Trachsel et al., 2007). A study in Lithuania detected significant differences between a modified McMaster method and this sieving-flotation technique (Bružinskaitė et al., 2009). Using the latter technique, the prevalence for taeniids was almost three times higher. The use of a highly sensitive method is important especially for Echinococcus species which tend to produce lower egg loads given their lower biotic potential (Gemmell et al., 2001). Moreover, identification of Taenia species and Echinococcus strains from the isolated eggs allows to trace the sources of infection as well as to clarify the life cycle of the parasites, as it was done in this study.
It can be concluded that the Iberian wolf is a wild definitive host for E. intermedius in the Iberian Peninsula. Nevertheless, it should be stressed that, according to the current knowledge, the most important definitive host for Echinococcus spp. in this region remains the domestic dog. Regular deworming of dogs should therefore be a crucial feature of taeniid control. Further research on wild intermediate hosts and humans will be useful in clarifying the transmission patterns of these parasites in Portugal, as well as the importance of their wild cycle.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
To Francisco Álvares, Helena Rio Maior and Mónia Nakamura (CIBIO – University of Porto) for guidance and support on the collection and identification of many fecal samples. Also, to Daniel Hegglin and Alexander Mathis (IPZ-UZH) for the precious comments on the manuscript.
This work represents part of the master thesis of Diogo Guerra, veterinarian.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-Share Alike License, which permits noncommercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Álvares F., Pereira E., Petrucci-Fonseca F. O Lobo no Parque internacional Gerês-Xurés. Situação populacional, aspectos ecológicos e perspectivas de conservação. Galemys. 2000;12:223–239. [Google Scholar]
- Beato S., Parreira R., Calado M., Grácio M.A.A. Apparent dominance of the G1–G3 genetic cluster of Echinococcus granulosus strains in the central inland region of Portugal. Parasitol. Int. 2010;59:638–642. doi: 10.1016/j.parint.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Bružinskaitė R., Šarkūnas M., Torgerson P.R., Mathis A., Deplazes P. Echinococcosis in pigs and intestinal infection with Echinococcus spp. in dogs in southwestern Lithuania. Vet. Parasitol. 2009;160:237–241. doi: 10.1016/j.vetpar.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Carreira R.S., Petrucci-Fonseca F. Lobo na região oeste de Trás-os-Montes (Portugal) Galemys. 2000;12:223–239. [Google Scholar]
- Carvalho-Varela M., Marcos M.V.M. A helmintofauna da raposa (Vulpes vulpes silacea, Miller 1907) em Portugal. Acta Parasitol. Port. 1993;1:73–79. [Google Scholar]
- Castro A., Silva M., Veloso G., Freire L., Rodrigues A., Silva E., Agante A., Conceição M.A.P., Correia da Costa J.M. Determination of Echinococcus granulosus genotypes with NADH dehydrogenase 1 and cytochrome c oxidase 1 sequences. Acta Parasitol. Port. 2005;12:395–396. [Google Scholar]
- Craig H.L., Craig P.S. Helminth parasites of wolves (Canis lupus): a species list and an analysis of published prevalence studies in Nearctic and Palaearctic populations. J. Helminthol. 2005;79:95–103. doi: 10.1079/joh2005282. [DOI] [PubMed] [Google Scholar]
- David de Morais J.A. The rise and decline of human hydatid disease in Portugal: historical and epidemiological analysis. Med. Int. 2010;17:246–256. [Google Scholar]
- Deplazes P., Dinkel A., Mathis A. Molecular tools for studies on the transmission biology of Echinococcus multilocularis. Parasitology. 2003;127:S53–S61. doi: 10.1017/s0031182003003500. [DOI] [PubMed] [Google Scholar]
- Eckert J., Gottstein B., Heath D., Liu F.-J. Prevention of Echinococcosis in humans and safety precautions. In: Eckert J., Gemmell M.A., Meslin F.-X., Pawłowski Z.S., editors. Manual on echinococcosis in humans and animals: a public health problem of global concern. OIE and WHO; Paris, France: 2001. pp. 238–248. [Google Scholar]
- Gemmell M.A., Roberts M.G., Beard T.C., Lawson J.R. Epidemiology. In: Eckert J., Gemmell M.A., Meslin F.-X., Pawłowski Z.S., editors. Manual on echinococcosis in humans and animals: a public health problem of global concern. OIE and WHO; Paris, France: 2001. pp. 143–194. [Google Scholar]
- Hirvelä-Koski V., Haukisalmi V., Kilpelä S.-S., Nylund M., Koski P. Echinococcus granulosus in Finland. Vet. Parasitol. 2003;111:175–192. doi: 10.1016/s0304-4017(02)00381-3. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J. Echinococcus granulosus in Australia, widespread and doing well! Parasitol. Int. 2006;55(Suppl.):S203–S206. doi: 10.1016/j.parint.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Schurer J.M., Gesy K.M. Old problems on a new playing field: helminth zoonoses transmitted among dogs, wildlife, and people in a changing northern climate. Vet. Parasitol. 2011;182:54–69. doi: 10.1016/j.vetpar.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Mathis A., Deplazes P., Eckert J. An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J. Helminthol. 1996;70:219–222. doi: 10.1017/s0022149x00015443. [DOI] [PubMed] [Google Scholar]
- Moks E., Jõgisalu I., Saarma U., Talvik H., Järvis T., Valdmann H. Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. J. Wildl. Dis. 2006;42:359–365. doi: 10.7589/0090-3558-42.2.359. [DOI] [PubMed] [Google Scholar]
- Mwambete K.D., Ponce-Gordo F., Cuesta-Bandera C. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop. 2004;91:87–93. doi: 10.1016/j.actatropica.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Pimenta V., Barroso I., Álvares F., Correia J., Costa G., Moreira L., Nascimento J., Fonseca F., Roque S., Santos E. Relatório Técnico, Instituto de Conservação da Natureza/Grupo Lobo; Lisboa: 2005. Situação populacional do lobo em Portugal: resultado do censo nacional 2002/2003. pp. 158. [Google Scholar]
- Reiczigel, J., Rózsa, L., 2005. Quantitative Parasitology 3.0. Budapest. Distributed by the authors. Available online at: http://www.zoologia.hu/qp/qp.html. Access at 29 Dec 2012.
- Schneider R., Gollackner B., Schindl M., Tucek G., Auer H. Echinococcus canadensis G7 (pig strain): an underestimated cause of cystic echinococcosis in Austria. Am. J. Trop. Med. Hyg. 2010;82:871–874. doi: 10.4269/ajtmh.2010.09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia J.M., Torres J., Miquel J., Llaneza L., Feliu C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001;75(2):183–192. [PubMed] [Google Scholar]
- Silva M.S.S. Universidade Técnica de Lisboa, Faculdade de Medicina Veterinária,; Lisboa: 2010. Rastreio de parasitas gastrintestinais, pulmonares, cutâneos e musculares em canídeos domésticos e silvestres no norte de Portugal. Dissertação de Mestrado em Medicina Veterinária. pp. 119. [Google Scholar]
- Štefanić S., Shaikenov B.S., Deplazes P., Dinkel A., Torgerson P.R., Mathis A. Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol. Res. 2004;92:347–351. doi: 10.1007/s00436-003-1043-y. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A. The taxonomy, phylogeny and transmission of Echinococcus. Exp. Parasitol. 2008;119:439–446. doi: 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Torres J., Segovia J.M., Miquel J., Feliu C., Llaneza L., Petrucci-Fonseca F. Helmintofauna del lobo ibérico (Canis lupus signatus Cabrera, 1907). Aspectos potencialmente útiles en mastozoologia. Galemys. 2000;12:1–11. [Google Scholar]
- Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the feces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]


