Abstract
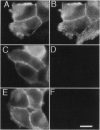
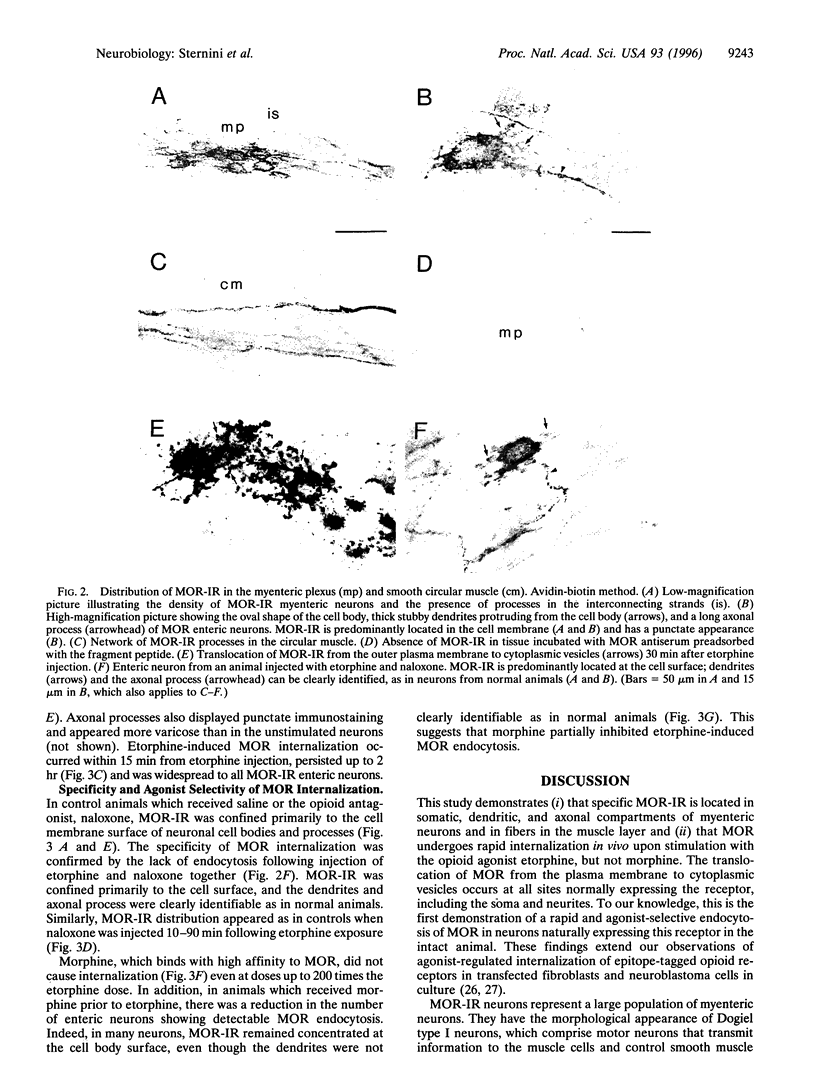
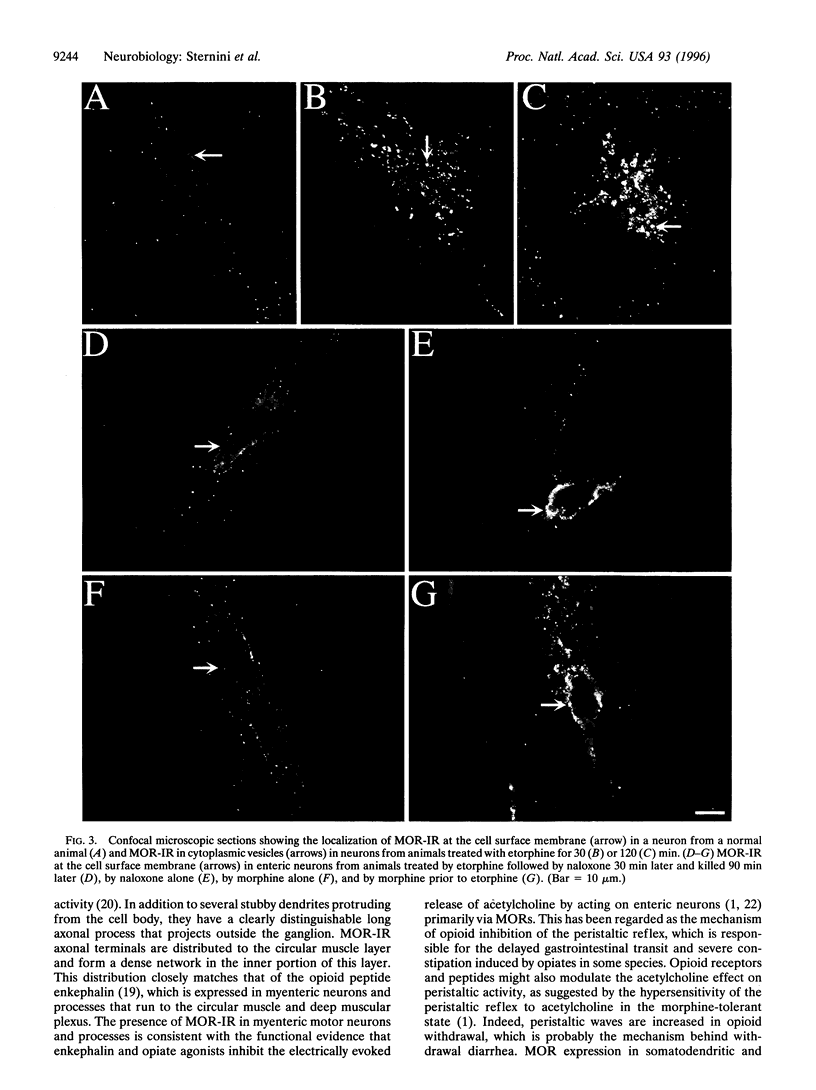
Opiate alkaloids are potent analgesics that exert multiple pharmacological effects in the nervous system by activating G protein-coupled receptors. Receptor internalization upon stimulation may be important for desensitization and resensitization, which affect cellular responsiveness to ligands. Here, we investigated the agonist-induced internalization of the mu opioid receptor (MOR) in vivo by using the guinea pig ileum as a model system and immunohistochemistry with an affinity-purified antibody to the C terminus of rat MOR. Antibody specificity was confirmed by the positive staining of human embryonic kidney 293 cells transfected with epitope-tagged MOR cDNA, by the lack of staining of cells transfected with the delta or kappa receptor cDNA, and by the abolition of staining when the MOR antibody was preadsorbed with the MOR peptide fragment. Abundant MOR immunoreactivity (MOR-IR) was localized to the cell body, dendrites, and axonal processes of myenteric neurons. Immunostaining was primarily confined to the plasma membrane of cell bodies and processes. Within 15 min of an intraperitoneal injection of the opiate agonist etorphine, intense MOR-IR was present in vesicle-like structures, which were identified as endosomes by confocal microscopy. At 30 min, MOR-IR was throughout the cytoplasm and in perinuclear vesicles. MOR-IR was still internalized at 120 min. Agonist-induced endocytosis was completely inhibited by the opiate antagonist naloxone. Interestingly, morphine, a high-affinity MOR agonist, did not cause detectable internalization, but it partially inhibited the etorphine-induced MOR endocytosis. These results demonstrate the occurrence of agonist-selective MOR endocytosis in neurons naturally expressing this receptor in vivo and suggest the existence of different mechanisms regulating cellular responsiveness to ligands.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden J. J., Garland A. M., Baluk P., Lefevre P., Grady E. F., Vigna S. R., Bunnett N. W., McDonald D. M. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Pizarro S., Ahuja M., Belmonte E., Blanchard N., Stadel J. M., Hoxie J. A. Changes in the structure and function of the human thrombin receptor during receptor activation, internalization, and recycling. J Biol Chem. 1994 Jan 28;269(4):2943–2952. [PubMed] [Google Scholar]
- Caron M. G., Lefkowitz R. J. Catecholamine receptors: structure, function, and regulation. Recent Prog Horm Res. 1993;48:277–290. doi: 10.1016/b978-0-12-571148-7.50014-2. [DOI] [PubMed] [Google Scholar]
- Di Chiara G., North R. A. Neurobiology of opiate abuse. Trends Pharmacol Sci. 1992 May;13(5):185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Miller R. J. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):653–664. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- Grady E. F., Garland A. M., Gamp P. D., Lovett M., Payan D. G., Bunnett N. W. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Mol Biol Cell. 1995 May;6(5):509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Ahuja M., Belmonte E., Pizarro S., Parton R., Brass L. F. Internalization and recycling of activated thrombin receptors. J Biol Chem. 1993 Jun 25;268(18):13756–13763. [PubMed] [Google Scholar]
- Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988 Jun;40(2):121–162. [PubMed] [Google Scholar]
- Loh H. H., Tao P. L., Smith A. P. Role of receptor regulation in opioid tolerance mechanisms. Synapse. 1988;2(4):457–462. doi: 10.1002/syn.890020414. [DOI] [PubMed] [Google Scholar]
- Lutz R. A., Pfister H. P. Opioid receptors and their pharmacological profiles. J Recept Res. 1992;12(3):267–286. doi: 10.3109/10799899209074796. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Allen C. J., Ghilardi J. R., Rogers S. D., Mantyh C. R., Liu H., Basbaum A. I., Vigna S. R., Maggio J. E. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., DeMaster E., Malhotra A., Ghilardi J. R., Rogers S. D., Mantyh C. R., Liu H., Basbaum A. I., Vigna S. R., Maggio J. E. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995 Jun 16;268(5217):1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo A., Evans C. J., Gundersen C. B. Antipeptide antibodies against a Torpedo cysteine-string protein. J Neurochem. 1994 Mar;62(3):873–880. doi: 10.1046/j.1471-4159.1994.62030873.x. [DOI] [PubMed] [Google Scholar]
- Minami M., Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. 1995 Sep;23(2):121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Hope B. T., Widnell K. L. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993 Dec;11(6):995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- Olson G. A., Olson R. D., Kastin A. J. Endogenous opiates: 1987. Peptides. 1989 Jan-Feb;10(1):205–236. doi: 10.1016/0196-9781(89)90098-3. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W. Pharmacological mechanisms of opioid analgesics. Clin Neuropharmacol. 1993 Feb;16(1):1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Pei G., Kieffer B. L., Lefkowitz R. J., Freedman N. J. Agonist-dependent phosphorylation of the mouse delta-opioid receptor: involvement of G protein-coupled receptor kinases but not protein kinase C. Mol Pharmacol. 1995 Aug;48(2):173–177. [PubMed] [Google Scholar]
- Pippig S., Andexinger S., Lohse M. J. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995 Apr;47(4):666–676. [PubMed] [Google Scholar]
- Raynor K., Kong H., Chen Y., Yasuda K., Yu L., Bell G. I., Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994 Feb;45(2):330–334. [PubMed] [Google Scholar]
- Reisine T., Bell G. I. Molecular biology of opioid receptors. Trends Neurosci. 1993 Dec;16(12):506–510. doi: 10.1016/0166-2236(93)90194-q. [DOI] [PubMed] [Google Scholar]
- Sim L. J., Selley D. E., Dworkin S. I., Childers S. R. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996 Apr 15;16(8):2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J. Opioid receptors and endogenous opioid peptides. Med Res Rev. 1991 Jul;11(4):357–374. doi: 10.1002/med.2610110402. [DOI] [PubMed] [Google Scholar]
- Sternini C., Anderson K. Calcitonin gene-related peptide-containing neurons supplying the rat digestive system: differential distribution and expression pattern. Somatosens Mot Res. 1992;9(1):45–59. doi: 10.3109/08990229209144762. [DOI] [PubMed] [Google Scholar]
- Sternini C. Structural and chemical organization of the myenteric plexus. Annu Rev Physiol. 1988;50:81–93. doi: 10.1146/annurev.ph.50.030188.000501. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Childers S., Pasternak G. An opiate-receptor gene family reunion. Trends Neurosci. 1994 Mar;17(3):89–93. doi: 10.1016/0166-2236(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Waterfield A. A., Smokcum R. W., Hughes J., Kosterlitz H. W., Henderson G. In vitro pharmacology of the opioid peptides, enkephalins and endorphins. Eur J Pharmacol. 1977 May 15;43(2):107–116. doi: 10.1016/0014-2999(77)90123-6. [DOI] [PubMed] [Google Scholar]
- Yu S. S., Lefkowitz R. J., Hausdorff W. P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993 Jan 5;268(1):337–341. [PubMed] [Google Scholar]
- von Zastrow M., Kobilka B. K. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992 Feb 15;267(5):3530–3538. [PubMed] [Google Scholar]