Abstract
Background
Resistance to broad-spectrum beta lactams mediated by extended spectrum beta lactamases (ESBLs) and AmpC beta lactamases (AmpC βLs) enzymes is an increasing problem worldwide. Determination of their prevalence is essential to formulate an effective antibiotic policy and hospital infection control measures. Present study was undertaken to determine the prevalence of ESBL and AmpC βL producers in ICU of a tertiary care center.
Methods
A total of 262 clinical isolates comprising of Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis that were recovered from various clinical specimens over a one year period, were studied. Antibiogram profile was determined to conventionally used antibiotics, along with recommended tests for detection of ESBL and AmpC βL production.
Results
40.07% (105/262) were found to be ESBL producers, 14.8% (39/262) were AmpC bL producers. The coexistence of ESBL and AmpC βL producers was detected in 9.9% (26/262) of the isolates.
Conclusion
Screening of multidrug resistant bacteria especially belonging to the Enterobacteriaceae poses considerable therapeutic challenges in critical care patients because of the production of ESBL and AmpC βL. Strategies to keep a check on the emergence of such drug resistant microbes by hospital environmental surveillance and laboratory monitoring should form an important aspect of Hospital Infection control policy guidelines.
Keywords: Extended spectrum beta lactamase, AmpC beta lactamase, Escherichia coli, Klebsiella pneumoniae
Introduction
The rapid emergence of antibiotic resistance among the hospital pathogens is a serious threat to the management of infectious diseases. β-lactam antibiotics are the most frequently used antimicrobials for empirical therapy. Production of β-lactamases is one of the strategies adopted by bacteria to develop resistance to β-Lactam class of antibiotics. The first plasmid mediated β-lactamase: TEM-1 (Temoniera-1) was reported in 1965 from an Escherichia coli isolated from a patient in Greece. Since then the TEM-1 β-lactamase has spread worldwide in different species of bacteria. Another plasmid mediated β-lactamase found in Klebsiella pneumoniae and E. coli is SHV-1 (sulfhydryl “variable”).1 The introduction of the third generation cephalosporins into clinical practice in the early 1980s was considered as a major breakthrough to fight against such β-lactamases producers. Soon after that, the first report of plasmid encoded β-lactamase capable of hydrolyzing the extended spectrum cephalosporins was published in 1983 from Germany.2 These new β-lactamases termed Extended spectrum beta lactamases (ESBLs), commonly involved in nosocomial infections, are derived from mutation in older beta-lactamases like (TEM-1, TEM-2 and SHV-1). ESBLs are enzymes that mediate resistance to extended spectrum cephalosporins (third generation cephalosporin, 3GCs) and monobactams (aztreonam) but do not affect cefamycins (cefoxitin, cefotetan, cefmetazole, flomoxef) or carbapenems (imipenem, meropenem, ertapenem, doripenem etc). They are inhibited by β-Lactamase inhibitor combinations (BLIs) such as clavulanic acid, sulbactam and tazobactam. Therefore, any strain resistant to 3GC but sensitive to β-Lactam/β-lactam inhibitor combination (BL/BLI) is likely to contain ESBL. ESBLs are encoded by transferable conjugative plasmids, which are responsible for the dissemination of resistance to other gram negative bacteria in a hospital and in the community.2 ESBLs are most commonly produced by Klebsiella spp. and E. coli. However, Enterobacter, Salmonella, Proteus, Citrobacter, Morganella, Serratia, Shigella, Pseudomonas and Burkholderia spp. also produce them.
AmpC beta-lactamases (AmpC βLs) first reported in 1970's3 usually confers on the bacterium, resistance to penicillins, cephalosporins, cephamycins and monobactams. The organisms develop resistance to BL/BLI combinations but are usually sensitive to the carbapenems. This lack of inhibition by cephamycins and β-lactamase inhibitors differentiates AmpC βL producers from the ESBL producers. Mechanism of drug resistance in AmpC βL can be chromosomal or plasmid mediated. Chromosomal mediated resistance is due to mutation in the nucleotide sequence at some point of the DNA of the bacteria and such genes are not easily transferable to other bacterial species. Plasmid mediated AmpC βLs have arisen by the transfer of chromosomal genes for AmpC β-lactamase onto plasmids. These genetic determinants can spread laterally and to other bacteria through lateral transfer of plasmids. Majority of AmpC βLs are chromosomally mediated (Unlike ESBLs which are Plasmid mediated) and are found in SPACE bugs (Serratia, Pseudomonas, Acinetobacter, Citrobacter and Enterobacter spp.). Plasmid mediated AmpC βLs are seen in isolates of E. coli, K. pneumoniae, Salmonella spp., Citrobacter freundii, Enterobacter aerogenes, and Proteus mirabilis.3,4
Recently, Gram negative organisms that produce both ESBLs and AmpC βLs are being increasingly reported worldwide.5 These organisms usually exhibit multidrug resistance that is not always detected in routine antimicrobial susceptibility tests. It is necessary to know their prevalence in a hospital setting so as to enable the clinician to select appropriate antibiotic regimens at the earliest to reduce average length of stay in a hospital there by reducing health-care costs and to formulate an effective antibiotic policy. The inability to detect such complex resistance phenotypes is a serious challenge and a major determinant in the uncontrolled spread of ESBL-producing organisms and related treatment failures in a hospital setting.5
Materials and methods
A prospective study was conducted over a period of one year (January to December 2009) with an aim to detect the prevalence of ESBL and AmpC βL producing strains in the intensive care unit of a large tertiary care center of Armed Forces Medical Services.
Bacterial strains
The study was conducted on consecutive non-duplicate isolates of E. coli, K. pneumoniae and P. mirabilis isolated from different clinical specimens such as urine, pus, blood and body fluids. The study was restricted to these isolates since Clinical Laboratory Standards Institute (CLSI) recommends ESBL testing and reporting only for these organisms.6 Bacterial identification was performed by routine conventional microbial culture and biochemical tests using standard recommended techniques.7
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing and interpretation for all these isolates was conducted on Mueller Hinton agar (HiMedia, Mumbai, India) by the standard disc diffusion method as per CLSI guidelines using discs of standard potency.6 The antibiotics tested were as follows (potency in μg/disc): ceftazidime (30), cefotaxime (30), cefepime (30), cefoxitin (30), ceftriaxone (30), piperacillin (100), amikacin (30), netilmicin (30), gentamicin (10) ciprofloxacin (5), piperacillin/tazobactam (100/10), ticarcillin/clavulanic acid (75/10), meropenem (10) and imipenem (10).
ESBL detection
All isolates showing reduced susceptibility to ceftazidime (zone diameter of ≤22 mm), ceftriaxone (zone diameter of ≤25 mm) or cefotaxime (zone diameter of ≤27 mm) as recommended by CLSI guidelines, were selected for confirmation of ESBL production. Isolates were tested for ESBL by standard CLSI double-disc diffusion method and double disc synergy test and using E test (AB Biodisk, Solna, Sweden) for detecting the MIC. These tests were checked for quality using standard control ESBL negative strain of E. coli ATCC 25922.
CLSI disc method
For the CLSI disc method,6 ceftazidime (30 μg) discs were used, with and without clavulanate (10 μg). ESBL production was indicated by an increase in zone size of 5 mm or more in the disk with ceftazidime and clavulanic acid combination as compared to the disc of ceftazidime alone [Fig. 1].
Fig. 1.
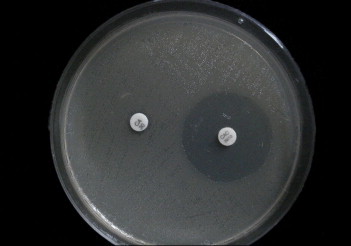
Phenotypic confirmation test of an ESBL producing strain showing zone size of more than 5 mm in the disk with ceftazidime and clavulanic acid (CAC) as compared to Ceftazidime (CA).
Double disc synergy test (DDST)
Synergy between a disc of third generation cephalosporin such as cefotaxime, ceftriaxone or ceftazidime (30 μg) and ceftazidime/clavulanic acid (30/10 μg) disc was seen.6 Mueller Hinton agar plates were prepared and inoculated with standardized inoculums of the bacteria (0.5 McFarland standard) to form a lawn culture. A 30 μg disc of each 3GC antibiotic was placed on the agar at a distance of 15 mm (centre to centre) from a ceftazidime/clavulanic acid disc. E. coli ATCC 25922 was used as the negative control and an in-house ESBL producer was used as the positive control. ESBL production was interpreted as positive if the inhibition zone around the test antibiotic disc increased toward the ceftazidime/clavulanic acid disc [Fig. 2].
Fig. 2.
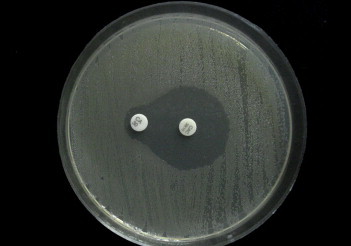
Double disc synergy test showing the inhibition zone around Ceftazidime disc (CA) increasing toward the Ceftazidime plus Clavulanic acid disc (CAC), confirming an ESBL producer.
E tests for ESBLs detection
The ceftazidime/ceftazidime-clavulanate (TZ-TZL) ESBL E test strip generates a stable concentration gradient of ceftazidime (MIC test range, 0.5–32 mg/L) on one end and the remaining end generates a gradient of ceftazidime (MIC test range, 0.064–4 mg/L) plus 4 mg/L clavulanic acid. Similarly, the cefotaxime/cefotaxime-clavulanate (CT-CTL) E test ESBL strip contains cefotaxime (MIC test range, 0.25–16 mg/L) and cefotaxime (MIC test range, 0.016–1 mg/L) plus 4 mg/L clavulanic acid. The E test procedure, reading, and interpretation were performed according to the manufacturer's instructions.8 Isolated colonies from an overnight plate were suspended in saline (0.85% NaCl) to achieve an inoculum equivalent to 0.5 McFarland standard. This suspension was swabbed on a Mueller Hinton agar plate and allowed to dry completely. An ESBL E test strip was then applied to the agar surface with sterile forceps and the plate was incubated at 35 °C overnight. ESBL results were read either as MIC values or observation of “phantom zones” or deformation of inhibition ellipses. Reduction of MIC by 3 log2 dilutions or MIC ratio ≥8 in the presence of clavulanic acid is indicative of ESBL production. Deformation of ellipses or the presence of a “phantom zone” is also indicative of ESBL production even if the MIC ratio is <8 or cannot be read [Fig. 3].
Fig. 3.
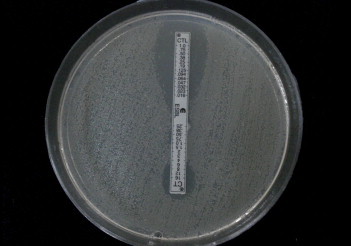
E test for ESBL confirmation showing a ratio more than 8 in MIC value of Cefotaxime (CT)/Cefotaxime + Clavulanic acid (CTL); 4/<.016.
Test for AmpC β-lactamase detection
All isolates showing reduced susceptibility to ceftazidime, ceftriaxone, cefotaxime or cefoxitin (30 μg) (zone diameter ≤18 mm) were tested for the presence of AmpC βL enzyme by AmpC E test. The cefotetan/cefotetan-cloxacillin (CN/CNI) AmpC strip contains cefotetan (MIC test range, 0.5–32 mg/L) and cefotetan (MIC test range, 0.5–32 mg/L) plus cloxacillin. The E test procedure, reading, and interpretation were performed according to the manufacturer's instructions.8 Reduction of MIC by 3 log2 dilutions or MIC ratio ≥8 in the presence of cloxacillin is indicative of AmpC production [Fig. 4].
Fig. 4.
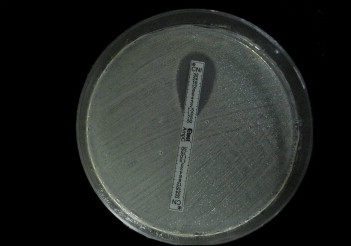
E test for AmpC producers showing a ratio more than 8 in MIC value of Cefotetan(CN)/Cefotetan + Cloxacillin (CNI); >32/.75.
Results
A total of 262 isolates of E. coli (n = 141), Klebsiella spp. (n = 114) and P. mirabilis (n = 07) were recovered from different clinical samples comprising of urine, pus, blood and body fluids. The total of potential ESBL producers showing reduced susceptibility to 3GCs was 154. Confirmatory tests for ESBL production were performed subsequently on these 154 isolates.
Out of 154 isolates, 101 isolates were found to be ESBL producers by phenotypic confirmatory tests using CLSI disc and DDST method and 105 (40.07%) isolates were found to be ESBL producers by E test [Table 1].
Table 1.
Results of screening and confirmatory tests for ESBL Production.
| Organism | Total no of isolates | No of isolates showing resistance to 3GCs in screening test | No of isolates positive in Double disc synergy test | No of isolates positive by E test |
|---|---|---|---|---|
| E. coli | 141 | 89 | 58 | 62 |
| K. pneumoniae | 114 | 63 | 43 | 43 |
| Proteus mirabilis | 7 | 2 | Nil | Nil |
| Total | 262 | 154 | 101 | 105 |
Out of the 141 isolates, 44% (62/141) of E. Coli, and out of 114 isolates, 32% (43/114) of Klebsiella pneumonia were found to be ESBL producers. None of the strains of P. mirabilis was an ESBL producer. Distribution of ESBL positive isolates was highest amongst the urinary isolates accounting for 42% of the total isolates recovered [Table 2].
Table 2.
Distribution of ESBL & Pure AmpC βL positive isolates in different clinical samples.
| Clinical Sample | ESBL Producers in E. coli isolates | Pure AmpC producers in E. coli isolates | ESBL Producers in K. pneumoniae isolates | Pure AmpC producers in K. pneumoniae isolates | Total |
|---|---|---|---|---|---|
| Urine | 23 | 8 | 21 | 3 | 55 |
| Blood Culture | 18 | 1 | 11 | 1 | 31 |
| Body Fluids | 17 | – | 9 | – | 26 |
| Pus | 4 | – | 2 | – | 6 |
| 62 | 9 | 43 | 4 | 118 | |
Among the 105 ESBL-positive isolates detected by E test, 26 also tested positive for transferable AmpC βL and 79 were lone ESBL producers. Thus, co-production of ESBL and AmpC βL was observed in 26 (9.9%) isolates. AmpC βL alone was detected in an additional 13 isolates, the total number of AmpC producing isolates thus being 39 (14.8%). All AmpC producers were found to be cefoxitin resistant. An interesting but notable observation was that 11 isolates that were cefoxitin resistant were found to be negative for AmpC production by E test.
Antimicrobial sensitivity pattern
Multidrug resistance was significantly higher among β-lactamase producers than in non β-lactamase producers. All 118 β-lactamase producing isolates were sensitive to Imipenem. Resistance to various other antibiotics conventionally used in empirical therapy was amikacin (30%), netilmicin (41%), gentamicin (79.6%), ciprofloxacin (71.1%), piperacillin–tazobactam (24.5%), and ticarcillin–clavulanic acid (25.4%) [Table 3].
Table 3.
Antimicrobial resistance patterns of β lactamase producers and Non β lactamase producers.
| Antimicrobials | β lactamase producers (n = 118) No of isolates resistant |
Non β lactamase producers (n = 144) No of isolates resistant |
||
|---|---|---|---|---|
| ESBL Producers (n = 79) | ESBL & AmpC βL producer (n = 26) | Pure AmpC βL producer (n = 13) | ||
| Amikacin | 24 | 8 | 3 | 27 |
| Gentamicin | 66 | 19 | 9 | 64 (p < 0.01) |
| Netilmicin | 29 | 9 | 4 | 52 |
| Piperacillin | 61 | 17 | 9 | 52 (p < 0.01) |
| Cefotaxime | 71 | 24 | 10 | 55 (p < 0.01) |
| Ceftriaxone | 67 | 19 | 8 | 52 (p < 0.01) |
| Ceftazidime | 77 | 24 | 11 | 59 (p < 0.01) |
| Cefepime | 34 | 4 | 0 | 18 |
| Cefoxitin | 0 | 26 | 13 | 11 |
| Ciprofloxacin | 59 | 17 | 8 | 41 (p < 0.01) |
| Piperacillin – Tazobactam | 2 | 16 | 11 | 15 |
| Ticarcillin – Clavulanic acid | 2 | 17 | 11 | 19 |
| Imipenem | 0 | 0 | 0 | 0 |
Discussion
This study demonstrates the prevalence of ESBL mediated drug resistance to third generation cephalosporin by Gram negative bacilli belonging to the Enterobacteriaceae family in the critically ill patients admitted in Intensive Care Unit of a tertiary hospital. ESBL and AmpC βL detection is not routinely carried out in many microbiology units of service laboratories. This could be attributed to lack of awareness or lack of resources and facilities to conduct ESBL identification.
In the present study, the prevalence of ESBL producers was found to be 40.07% (105 out of 262) amongst E. coli and K. pneumoniae isolates. The alarming rate of resistance noted among these isolates in the present study, is of concern. Resistance of ESBL producing isolates to 3GCs was found to coexist with resistance to two or more antibiotics such as piperacillin (p < 0.01), ciprofloxacin (p < 0.01) and gentamicin (p < 0.01). This coexistence of multidrug resistance has been reported earlier.9,10 Mechanisms of co-resistance are not clear, but one possible mechanism is the co-transmission of ESBL and resistance to other antimicrobials within the same conjugative plasmids. The same has been demonstrated in a study by Mishra et al which showed plasmid mediated resistance in K. pneumoniae isolates to multiple antibiotics including cephalosporins and aminoglycosides.11 Within countries, hospital to hospital variability is usual. A large study from more than 100 European intensive care units (ICU) found that the prevalence of ESBLs in Klebsiellae ranging from as low as 3% in Sweden to as high as 34% in Portugal.12 In Turkey, a survey of Klebsiella spp. from ICUs from eight hospitals showed that 58% of 193 isolates harbored ESBLs.13 Moland and colleagues have shown that ESBL producing isolates were found in 75% of 24 medical centers in the United States.14 ESBLs have also been documented in Israel, Saudi Arabia, and a variety of North African countries.15–17 In China, ESBL producers vary between 25 and 40%.18 South East Asian countries reported presence of ESBLs in 5–8% of E. coli isolates from Japan, Korea, Malaysia and Singapore but in 12–24% of isolates from Thailand, Taiwan, Philippines and Indonesia.2 In India, the prevalence rate varies in different institutions from 28 to 84%.19 A study from Coimbatore, Tamil Nadu, showed the presence of ESBLs to be 40% while a study from Nagpur showed it as 50% from the urinary isolates.20,21 Another study in 2005, from New Delhi, showed 68.78% of the strains of gram negative bacteria to be ESBL producers.22 In our study the prevalence of ESBL in E. coli was 44% and in K. pneumoniae it was 32%.
Organisms over expressing AmpC βLs are of major clinical concern because these are usually resistant to all beta lactam antimicrobials, except for cefepime, cefpirome and carbapenems.23,24 In contrast to ESBLs, they hydrolyze cephamycins and are not inhibited by beta lactamase inhibitors. Constitutive over expression of AmpC occurs either by deregulation through the mutation of the AmpR gene in the chromosome or by acquisition of a transferable AmpC gene on a plasmid or on another transferable element commonly called as plasmid mediated AmpC beta lactamase.23,24 The origin of AmpC in E. coli is chromosomal, although recently, plasmid mediated AmpC also has been isolated. K. pneumoniae harbors only plasmid mediated AmpC. Detection of any type of AmpC βL is a challenge to clinical microbiologists since the bacteria show marked variations in the expression of the enzymes, making the task of laboratory detection more complicated. However, several studies have been done on various test methods namely, the three dimensional test, modified double disc test, AmpC disc test,25 inhibitor based method employing inhibitors like boronic acid and broth micro-dilution method.26 Despite the varied phenotypic tests available, isoelectric focusing and genotypic characterization27 are considered gold standard for detection. The accurate detection of plasmid mediated AmpC is important to improve the clinical management of infection and to provide sound epidemiological data.
There is a paucity of data from Indian laboratories on the coexistence of multiple beta lactamases in individual isolates. Studies from various parts of India have reported the prevalence of AmpC in clinical isolates of Enterobacteriaceae as varying from 2.2% to 20.7%.23,24 However, these studies were designed to estimate the prevalence of AmpC among all the clinical isolates of Enterobacteriaceae. AmpC βLs when present along with ESBLs can mask the phenotype of the latter.4 In this study, we found that both these enzymes were equally expressed suggesting a possible low level expression of AmpC enzymes. However, in all these AmpC producers, we were not able to distinguish between the chromosomal derepressed and plasmid mediated enzymes, as this requires genotypic confirmatory tests. Our study highlights the importance of appropriate detection methods for AmpC enzymes in those isolates, which are already designated to be ESBL positive. The coexistence of different classes of beta lactamases in a single bacterial isolate poses a challenge both in diagnosis and therapy. Use of a cefoxitin disc is useful in screening for AmpC. However, we observed that 28% (11 out of 39) cefoxitin resistant isolates did not produce AmpC. This may be attributable to other resistance mechanisms such as decreased porin entry channels or increase in efflux pump expression. The same has been demonstrated in a study by Ananthan and Subha which showed loss of a porin Omp K35 and OmpK36 in 50% isolates of Cefoxitin resistant K. pneumoniae and E. coli.28 Other studies in India have shown 19–27% AmpC-nonproducers which were found to be resistant to Cefoxitin.5,23 Loss of porins is found to augment resistance provided by ESBLs and plasmid mediated AmpC β-lactamases also leading to resistance to carbapenems. More extensive study related to OMP profiles and resistance patterns needs to be carried out to emphasize the clinical impact of porin mediated β-lactam resistance among the clinical isolates of Klebsiella spp. and E. coli.
Conclusion
In the present study, we found an alarming number of ESBL producing E. coli and K. pneumoniae strains which simultaneously produced AmpC beta lactamase. The Hospital laboratories should screen possible ESBL and AmpC producers by including 3GC, ceftazidime/clavulanic acid and cefoxitin discs along with the standard antibiotic discs as part of their protocol of testing Enterobacteriaceae. The laboratories should have the capacity to detect multiple beta lactamases that are already designated as ESBL producers, so that appropriate therapy can be chosen for patient management. The report must state whether the isolate is a suspected or proven ESBL producer. The report must also include a note that ESBL producer may result in therapeutic failure with antimicrobials such as penicillin, aztreonam and all cephalosporin except cephamycins irrespective of their in vitro susceptibility. ESBL testing should necessarily be carried out in all bacterial isolates showing resistance to the third generation cephalosporins and other β-lactam antimicrobials. Considering the gravity of the implication of wrong therapy in critical care, looking for ESBL and AmpC Beta lactamase producers must be made mandatory in all reporting in microbiology laboratories and clinicians also educated on the issue.
Intellectual contribution
Study concept: Col Naveen Grover, Brig AK Sahni
Drafting and manuscript revision: Col Naveen Grover, Brig AK Sahni
Statistical analysis: Col Naveen Grover, Brig AK Sahni
Study supervision: Col S Bhattacharya (Retd)
Conflicts of interest
All authors have none to declare.
References
- 1.Livermore D.M. Beta-lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson D.L., Bonomo R.A. Extended spectrum beta lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philippon A., Arlet G., Jacoby G.A. Plasmid-determined AmpC type beta lactamases. Antimicrob Agents Chemother. 2002;46(1):1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacoby G.A. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha P., Sharma R., Rishi S., Sharma T., Sood S., Pathak D. Prevalence of extended spectrum beta lactamase and AmpC beta lactamase producers among Escherichia Coli isolates in a large tertiary care hospital in Jaipur. Indian J Pathol Microbiol. 2008;51(3):367–369. doi: 10.4103/0377-4929.42512. [DOI] [PubMed] [Google Scholar]
- 6.CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2008. Performance Standards for Antimicrobial Susceptibility Testing. Approved Standards; Eighteenth Informational Supplement. M100-S18. [Google Scholar]
- 7.Collee J.G., Miles R.S., Wan B. Tests for the identification of bacteria. In: Collee J.G., Fraser A.G., Marmion B.P., Simmons A., editors. Mackie and Mc Cartney Practical Medical Microbiology. 14th ed. Churchill Livingstone; Edinburgh: 1996. pp. 131–150. [Google Scholar]
- 8.Stürenburg E., Sobottka I., Noor D., Laufs R., Mack D. Evaluation of a new cefepime-clavulanate ESBL E test to detect extended-spectrum β-lactamases in an Enterobacteriaceae strain collection. J Antimicrob Chemother. 2004;54:134–138. doi: 10.1093/jac/dkh274. [DOI] [PubMed] [Google Scholar]
- 9.Subha A., Ananthan S. Extended spectrum beta lactamase (ESBL) mediated resistance to third generation cephalosporins among Klebsiella pneumoniae in Chennai. Indian J Med Microbiol. 2002;20:92–95. [PubMed] [Google Scholar]
- 10.Duttaroy B., Mehta S. Extended spectrum β lactamases (ESBL) in clinical isolates of Klebsiella pneumoniae and Escherichia coli. Indian J Pathol Microbiol. 2005;48:45–48. [PubMed] [Google Scholar]
- 11.Mishra R., Kumar M., Menon P.K., Ohri V.C. Plasmid mediated antibiotic resistance in Klebsiella pneumoniae. Indian J Pathol Microbiol. 2001;44(4):427–429. [PubMed] [Google Scholar]
- 12.Hanberger H., Garcia-Rodriguez J.A., Gobernado M., Goossens H., Nilsson L.E., Struelens M.J. Antibiotic susceptibility among aerobic gram negative bacilli in intensive care units in 5 European countries. French and Portuguese ICU Study Groups. JAMA. 1999;281:67–71. doi: 10.1001/jama.281.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Gónseren F., Mamikottlu L., Oztórk S., Yócesoy M., Biberottlu K., Yulutt N. A surveillance study of antimicrobial resistance of gram-negative bacteria isolated from intensive care units in eight hospitals in Turkey. J Antimicrob Chemother. 1999;43:373–378. doi: 10.1093/jac/43.3.373. [DOI] [PubMed] [Google Scholar]
- 14.Moland E.S., Black J.A., Ourada J., Reisbig M.D., Hanson N.D., Thomson K.S. Occurrence of newer beta-lactamases in Klebsiella pneumoniae isolates from 24 US hospitals. Antimicrob Agents Chemother. 2002;46:3837–3842. doi: 10.1128/AAC.46.12.3837-3842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borer A., Gilad J., Menashe G., Peled N., Riesenberg K., Schlaeffer F. Extended spectrum beta-lactamase producing Enterobacteriaceae strains in community-acquired bacteremia in Southern Israel. Med Sci Monit. 2002;8:44–47. [PubMed] [Google Scholar]
- 16.El-Karsh T., Tawfik A.F., Al-Shammary F., Al-Salah S., Kambal A.M., Shibl A. Antimicrobial resistance and prevalence of extended spectrum beta-lactamase among clinical isolates of gram-negative bacteria in Riyadh. J Chemother. 1995;7:509–514. doi: 10.1179/joc.1995.7.6.509. [DOI] [PubMed] [Google Scholar]
- 17.AitMhand R., Soukri A., Moustaoui N. Plasmid-mediated TEM-3 extended-spectrum beta-lactamase production in Salmonella typhimurium in Casablanca. J Antimicrob Chemother. 2002;49:169–172. doi: 10.1093/jac/49.1.169. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y., Zhou W., Chen Y., Ding Y., Ma Y. Epidemiological and antibiotic resistant study on extended-spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae in Zhejiang Province. Chin Med J (Engl) 2002;115:1479–1482. [PubMed] [Google Scholar]
- 19.Das A., Ray P., Garg R., Kaur B. Proceedings of the Silver Jubilee Conference of IAMM. All India Institute of Medical Sciences; New Delhi: 2001. Extended spectrum beta-lactamase production in Gram negative bacterial isolates from cases of septicemia. [Google Scholar]
- 20.Babypadmini S., Appalaraju B. Extended spectrum β-lactamases in urinary isolates of Escherichia coli and Klebsiella pneumoniae-prevalence and susceptibility pattern in a tertiary care hospital. Indian J Med Microbiol. 2004;22:172–174. [PubMed] [Google Scholar]
- 21.Tankhiwale S.S., Jalgaonkar S.V., Ahamad S., Hassani U. Evaluation of extended spectrum beta lactamase in urinary isolates. Indian J Med Res. 2004;120:553–556. [PubMed] [Google Scholar]
- 22.Mohanty S., Singhal R., Sood S., Dhawan B., Das B.K., Kapil A. Comparative in vitro activity of beta-lactam/beta-lactamase inhibitor combinations against Gram negative bacteria. Indian J Med Res. 2005;122:425–428. [PubMed] [Google Scholar]
- 23.Manchanda V., Singh N.P. Occurrence and detection of AmpC beta-lactamases among Gram-negative clinical isolates using a modified three-dimensional test at Guru Tegh Bahadur Hospital, Delhi, India. J Antimicrob Chemother. 2003;51:415–418. doi: 10.1093/jac/dkg098. [DOI] [PubMed] [Google Scholar]
- 24.Ratna A.K., Menon I., Kapur I., Kulkarni R. Occurrence & detection of AmpC beta lactamases at a referral hospital in Karnataka. Indian J Med Res. 2003;118:29–32. [PubMed] [Google Scholar]
- 25.Pitout J.D., Reisbig M.D., Venter E.C., Church D.L., Hanson N.D. Modification of the double-disc test for detection of enterobacteriaceae producing extended-spectrum and AmpC beta-lactamases. J Clin Microbiol. 2003;41:3933–3935. doi: 10.1128/JCM.41.8.3933-3935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coudron P.E. Inhibitor-based methods for detection of plasmid mediated AmpC beta-lactamases in Klebsiella spp., Escherichia coli and Proteus mirabilis. J Clin Microbiol. 2005;43:4163–4167. doi: 10.1128/JCM.43.8.4163-4167.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Perez F.J., Hanson N.D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ananthan S., Subha A. Cefoxitin resistance mediated by loss of a porin in clinical strains of Klebsiella pneumoniae and Escherichia coli. Indian J Med Microbiol. 2005;23(1):20–23. doi: 10.4103/0255-0857.13867. [DOI] [PubMed] [Google Scholar]


