Abstract
The activating immunoreceptor NKG2D endows cytotoxic lymphocytes with the capacity to recognize and eliminate infected or malignant cells. The recognition of such harmful cells is enabled by binding of NKG2D to various MHC class I-related glycoproteins, which are upregulated in the course of viral infection or malignant transformation. The past years have witnessed substantial progress in our understanding of the mechanisms underlying the regulation of NKG2D ligands (NKG2DLs) by malignant cells, of tumor-associated countermeasures promoting escape from NKG2D-dependent immunosurveillance, and of therapeutic measures that may bolster the NKG2D/NKG2DL system against malignancies. Here, we summarize the current knowledge on the NKG2D/NKG2DL system and outline opportunities to exploit the tumoricidal function of NKG2D for anticancer immunotherapy.
Keywords: MIC, NK cell, NKG2D, Rae, T cell, ULBP, immune escape, tumor immunology
Preface
Cytotoxic lymphocytes are major protagonists in concepts of immunosurveillance and immunotherapy of cancer, as they are literally able to kill malignant cells. The selective killing of transformed cells requires a precise and unerring molecular recognition of “malignant self”, which represents a quite challenging task, especially in view of the highly diverse manifestations of cellular malignancy. Different subsets of cytotoxic lymphocytes including natural killer (NK) cells, CD8+ αβ T cells, and γδ T cells utilize distinct types of molecular recognition systems that detect malignant-self either directly (such as in the case of tumor-associated antigens) or indirectly (in the form of so-called “danger signals”).1 Nonetheless, all these lymphocytes (at least in humans) share the expression of NKG2D, which—upon binding to various stress-inducible NKG2D ligands (NKG2DLs)—stimulates effector responses.2-4 As the upregulation of NKG2DLs is linked to cellular processes that are associated with malignant transformation and NKG2DLs are frequently expressed on the surface of tumor cells,2,5-7 NKG2D has attracted vivid interest as a potential target for the development of novel immunotherapeutic anticancer regimens.
NKG2D is a Potent Activating Immunoreceptor of Immune Cells
NKG2D is composed of two disulfide-linked copies of a type II transmembrane glycoprotein that bears a single extracellular C-type lectin-like domain (CTLD), and hence is often referred to as a homodimeric C-type lectin-like receptor (CTLR).2,8 NKG2D monomers are encoded by the gene killer cell lectin-like receptor subfamily K, member 1 (KLRK1), which is embedded within a cluster of genes coding for immune-related CTLRs, called the natural killer gene complex (NKC).9,10 While NKG2D is relatively conserved among mammals, the number and types of NKG2DLs vary considerably among mammalian species. NKG2D-coding transcripts were first described and named together with the mRNAs coding for NKG2A, NKG2C, and NKG2E.11 However, as pointed out previously, the term NKG2D is misleading, as NKG2D is not related more closely to NKG2A, NKG2C and NKG2E, respectively, than to other NKC-encoded CTLRs.12 In 1999, the search for the receptor of the MHC class I-related orphan molecule MICA led to the functional characterization of NKG2D as a cell-surface receptor that stimulates the cytolytic response of human NK and T cells to stress-inducible MIC molecules.2 Subsequently, NKG2D was also shown to be expressed and to exert immunostimulatory functions in NK cells and activated CD8+ T lymphocytes of mice.13,14 In contrast to its mouse counterpart, human NKG2D is also expressed on the surface of naïve CD8+ T cells and even some CD4+ T cells that expand under specific pathophysiological conditions.2,15 Likewise, most γδ T cells and NKT cells express NKG2D and are activated by NKG2DLs.14,16 Very recently, the expression of NKG2D by some tumors (in situ) and malignant cell lines (in vitro) has been documented.17 It will be important to gain further insights into the functional relevance of NKG2D expressed by cancer cells in the context of tumor progression.
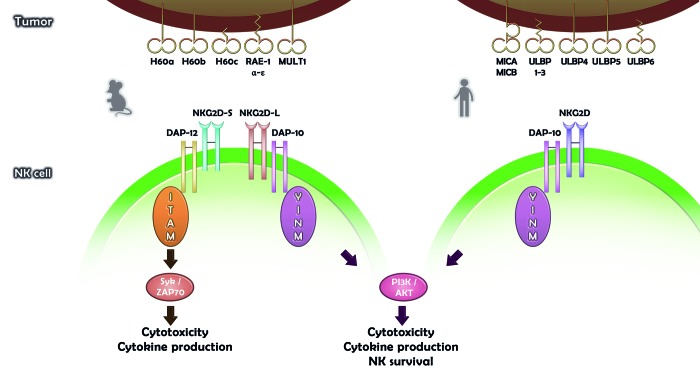
NKG2D is incapable of signal transduction by itself. Rather, the transmission of NKG2D-elicited signals requires the association of NKG2D with DAP10 forming together a hexameric structure containing two signaling dimers18,19 (Fig. 1). DAP10 is almost exclusively associated with NKG2D and contains motifs for the recruitment of phosphatidylinositol-3-kinase (PI3K) and GRB2/VAV1, which allow for the activation of downstream signaling by Akt and MAP kinases, respectively.18,20 Hence, the NKG2D-DAP10 receptor complex activates NK and T-cell cytotoxicity via the PI3K pathway and by recruiting VAV1.18,20 In mice, NKG2D exists in two distinct splice variants, a short (NKG2D-S) and a long (NKG2D-L) isoform, the latter of which contains 13 additional amino acids at the N-terminus.21,22 In activated mouse NK cells, NKG2D-S recruits immunoreceptor tyrosine-based activation motif (ITAM)-bearing DAP12, which enhances the reactivity mediated by NKG2D.21,22 In particular, the signaling cascade elicited by NKG2D-S and DAP12, which operates via the spleen tyrosine kinase (SYK) and ζ chain (TCR)-associated protein kinase 70kDa (ZAP70), not only triggers cytotoxicity, but also stimulates cytokine secretion21-23 (Fig. 1).
Figure 1. Diversity of NKG2D ligands and NKG2D signaling in mice and humans. Numerous NKG2D ligands (NKG2DLs) can be expressed on the surface of mouse (left) or human (right) malignant cells. In mice, NKG2D exists as a short (NKG2D-S) or a long (NKG2D-L) splice isoform. The activation of NKG2D-S promotes the cytotoxic function of natural killer (NK) cells as well as their capacity to secrete immunostimulatory cytokines. Upon engagement of NKG2D-S, SYK or ZAP70 are recruited through the ITAM-bearing adaptor DAP12. NKG2D-L and human NKG2D signal upon the recruitment/activation of DAP10, PI3K, AKT1, and GRB2/VAV1.
E Pluribus Ad Unum: Multiple NKG2DLs Bind One Receptor
The realm of NKG2DLs is surprisingly varied with regard to number, structure, and expression pattern. All NKG2DLs share an MHC class I-related α1α2 superdomain that – in spite of a substantial sequence diversity – constitutes the common site for interaction with NKG2D.24 Comparative structural analyses of several NKG2D/NKG2DL complexes revealed that promiscuous NKG2D binding is enabled by an interaction mode of rigid adaptation to structurally conserved patches in the highly diversified α1α2 superdomains.25,26 In humans, there are eight NKG2DLs, belonging to the MIC (i.e., MICA, MICB) and UL16-binding protein (i.e., ULBP1–6) families of MHC class I-related glycoproteins.4,27-29 Similarly, the mouse genome encodes multiple NKG2DLs belonging to various subfamilies of MHC class I-related molecules. These include retinoic acid early inducible gene 1 (Rae1)-like proteins (i.e., Rae1α, β, γ, δ, ε), members of the H60 protein family (i.e., H60a, b, c) as well as Mult14,30,31 (Fig. 1).
In general, NKG2DLs - unlike MHC class I molecules - do not bind β2-microglobulin or low molecular weight ligands, and are associated with the plasma membrane via transmembrane domains (such in the case of MICs, ULBP4, ULBP5, Mult1, H60a and H60b) or glycosylphosphatidylinositol (GPI) anchors (such in the case of ULBP1, ULBP2, ULBP3, ULBP6, Rae1 and H60c)4,29 (Fig. 1). The complexity of NKG2DLs is further increased by their polymorphic nature (particularly polymorphic are human MICA and MICB), their widely varying affinities for NKG2D, and their heterogeneous expression pattern.4,5,29
Owing to the variety and complexity of NKG2DLs, a comprehensive knowledge of the expression and regulation of these ligands is critical for a complete understanding of NKG2D functions.
Regulation of NKG2DLs
The expression of NKG2DLs is considered as an indicator of a state of cellular stress, for instance as it occurs in the course of infection or malignant transformation. In line with this notion, NKG2DLs are rarely found on the surface of healthy cells. Still, transcripts coding for MICs32 and some members of the ULBP family33 have been detected in several healthy tissues. An early report described the expression of MICA by cells of the gastrointestinal epithelium,34 but a comprehensive knowledge of NKG2DL expression at the protein level in healthy tissues is still missing. A recent report by the Raulet laboratory35 shows that E2F transcription factors, which play a major role in cell cycle entry, regulate the expression of mouse NKG2DLs. High rates of proliferation are not a prerogative of malignant cells but also occur during the development of normal tissues and in the course of tissue renewal. In this respect, Rae1 was shown to be expressed in the developing embryonic brain as well as in healing wounds.35 It can be assumed that, in the absence of cell stress or other pathological conditions, the elimination of NKG2DL-expressing cells is avoided as either NKG2DLs are expressed concomitantly with high levels of inhibitory ligands such as MHC class I molecules or as these cells are inaccessible to NK cells. The regulation of NKG2DL expression is a field of intense research that covers various transcriptional and post-transcriptional mechanisms affecting not only mRNA and protein stability, but also regulation of NKG2DL density on the cell surface.4,5,36,37 (Fig. 2). Besides being regulated by cell-intrinsic mechanisms, the expression of NKG2DLs is greatly influenced by factors released in the tumor microenvironment, such as interferons.4,29 Here, we will focus on the mechanisms of NKG2DL regulation that are of clinical relevance for anticancer immunotherapy.
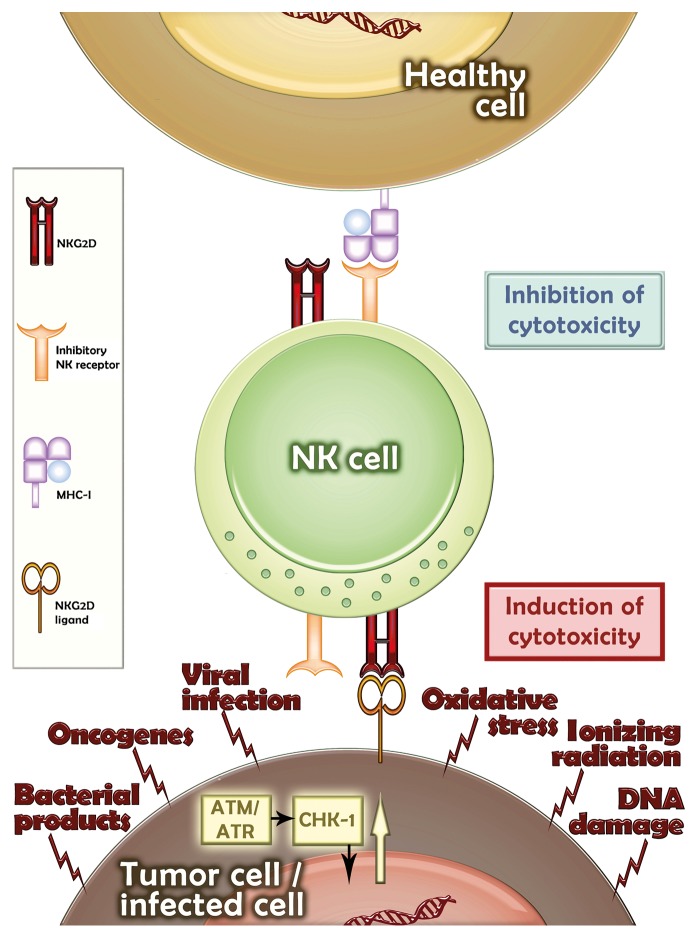
Figure 2. Upregulation of NKG2D ligands in transformed cells. Healthy cells generally do not express NKG2D ligands (NKG2DLs) and suppress the reactivity of natural killer (NK) cells by delivering inhibitory signals. In contrast, various stress conditions promote the upregulation of NKG2DLs in malignant or infected cells. ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia- and Rad3-related protein kinases; CHK-1, checkpoint kinase 1.
In 2005, Raulet and colleagues identified the DNA damage response mediated by the ataxia telangiectasia mutated (ATM) and (ataxia telangiectasia- and Rad3-related) ATR protein kinases as a key mechanism for the upregulation of NKG2DL on mouse and human tumor cells.38 Subsequently, human NKG2DLs were detected on cells that underwent senescence upon the activation of the DNA damage response.39 ATM as well as ATR are capable of activating the transcription factor TP53 (commonly known as p53), which is mutated or inactivated in a large fraction of human tumors. Using cellular systems of p53 induction, Textor et al. demonstrated that p53 can transactivate ULBP1 and ULBP2, but not the genes coding for other human NKG2DLs.40 Of importance, small p53-stabilizing compounds that are currently tested in clinical trials, such as ‘reactivation of p53 and induction of tumor cell apoptosis’ (RITA), have been shown to upregulate the expression of ULBP2 on the surface of some malignant cell lines,40 resulting in enhanced killing by NK cells.41 Accordingly, in a murine tumor model, the inducible genetic reactivation of p53 in malignant cells drove not only cellular senescence but also disease regression, which was partially dependent on the presence of NK cells.42 This study did not specifically address whether the NKG2D/NKG2DL system was involved in anticancer immunity, and presumably other factors produced by senescent cells were involved. Importantly, the upregulation of NKG2DLs by DNA damage can also occur in the absence of p53,38 pointing to the existence of p53-independent mechanisms of NKG2DL regulation. Low doses of DNA-damaging chemotherapeutic or radiotherapeutic regimens were shown to increase the expression of NKG2DLs on the surface of different cancer cell lines.43,44 Similarly, low doses of proteasome inhibitors such as bortezomib induced the expression of NKG2DLs in an ATM- and ATR-dependent fashion.39,45 The activation of NK cells might therefore contribute to the clinical benefits provided by these therapeutic regimens. Thus, combinatorial regimens incorporating low-dose chemo- or radiotherapy together with interventions that promote the activation of NK cells via NKG2D might improve current protocols of anticancer immunotherapy.
The expression of NKG2DLs is also regulated by histone deacetylases (HDACs), a class of enzymes that control key cellular processes including proliferation, survival and motility. HDAC inhibitors (HDACis) were shown to upregulate the expression of NKG2DLs on the surface of some cancer cells, promoting their NKG2D-dependent killing.46-48 Of note, we have evidence that HDACis also downregulate expression of B7-H6, a ligand of the activating NK cell receptor NKp30.49 Thus, the net response of NK cells to HDACi-treated targets depends on the relative contribution of NKG2D- vs. NKp30-dependent signaling pathways. Thus, potential combinatorial regimens involving HDACis and NK cell-based therapies should take such a differential regulation of activating NK cell ligands by tumor cells into consideration. There is increasing evidence that the expression of some NKG2DLs is under the control of cancer-relevant microRNAs.37 In this respect, it has been reported that the metastasis-associated microRNA (metastamir) miR-10b directly downregulates MICB, linking metastatic dissemination with the escape of malignant cells from NK-mediated immunosurveillance.50 In addition, the oncosuppressive microRNAs miR-34a and miR-34c repress ULBP2 in a p53-dependent manner, as recently documented in some human cancer cell lines.51 Thus, the microRNA expression pattern might greatly influence the recognition of malignant cells by NK cells via the NKG2D/NKG2DL signaling axis.
Post-transcriptional mechanisms of regulation, including those mediated by miRNAs, are likely to account for the discrepancy between the levels of NKG2DLs transcripts and NKG2DL cell surface expression. Another of such mechanisms is the shedding of NKG2DLs by malignant cells that contributes to reduced cell surface expression, as originally reported for MICA.52,53 Tumor cells shed some NKG2DLs from the cell surface owing to the activity of metalloproteases such as ADAM metallopeptidase domain 10 (ADAM10) and ADAM17, while other NKG2DLs are released in exosomes.4,29,54,55 Accordingly, many cancer patients exhibit increased circulating levels of soluble NKG2DLs.53,54 Some studies have shown that soluble NKG2DLs downregulate NKG2D expression, thereby impairing the NKG2D-mediated recognition of tumor cells by cytotoxic lymphocytes.52,54,80 The potential prognostic value of the circulating levels of soluble NKG2D ligands is discussed below.
Functional Significance of the NKG2D/NKG2DL System and Insights From Mouse Models
Up to now, numerous studies have addressed the function of NKG2D/NKG2DLs in vitro and in vivo, supporting the initial notion that this constitutes a peculiar immunosurveillance system for the recognition and elimination of potentially harmful (i.e., stressed, infected or malignant) cells by cytotoxic lymphocytes. Particularly suggestive are the numerous viral glycoproteins specifically dedicated to the retention or degradation of NKG2DLs, representing a countermeasure against the induction of NKG2DLs upon viral infection.56,57 Such an interplay between so-called “viral immunoevasins” and NKG2DLs provides a conceptual framework for explaining the diversity of NKG2DLs in the context of the evolutionary selection pressure exerted by viruses. In addition, the complexity of the NKG2DL system may have evolved by the necessity for tissue-specific or insult-specific immune responses.
The relevance of the NKG2D/NKG2DL system for cancer immunosurveillance has first been hypothesized when the expression of NKG2DLs was found to be associated with malignancy.58 Subsequently, the spontaneous or therapy-elicited expression of NKG2DLs on tumor cells, triggering their recognition and elimination by NK cells, has been documented in vitro and in animal studies.2,59-61 These studies also demonstrated that overexpression of NKG2DLs on cancer cells leads to tumor rejection irrespective of MHC class I molecules, but in a perforin-dependent manner.2,59-61 Importantly, the NKG2D-dependent cytotoxic activity of NK cells turned out not to be restricted to transformed cells, as NKG2DL-expressing splenocytes could be equally eliminated.62,63
Mice treated with neutralizing anti-NKG2D antibodies exhibited a significantly increased incidence of carcinogen-induced fibrosarcoma.64 Sarcoma cell lines derived from control mice expressed low (if not undetectable) levels of NKG2DLs such as Rae1, whereas sarcomas derived from animals treated with anti-NKG2D antibodies exhibited medium to high levels of Rae1, a phenomenon that was ascribed to tumor editing by NKG2D-expressing lymphocytes.65 Along similar lines, sarcoma cells from perforin-deficient mice also exhibited elevated expression levels of Rae1. These observations not only underline the impact of the perforin system in NKG2D-mediated cytotoxicity, but also lend further support to the tumor editing concept.65
Further evidence for a role of NKG2D in cancer immunosurveillance was obtained from NKG2D-deficient (Klrk1−/−) mice, which turned out to be more prone to develop various types of spontaneous cancer than NKG2D-sufficient mice.66 A detailed analysis of these mice revealed subtle changes in the receptor repertoire and maturation profile of NK cells, while the overall development and NKG2D-independent functions of NK cells appeared unaffected.67 Using another NKG2D-deficient mouse model, Polic and coworkers observed a decreased number of splenocytes, relatively pronounced alterations in NK cell subpopulations and an increased NK cell proliferation rate, suggesting a regulatory role for NKG2D in NK cell development. These NKG2D-deficient mice were also more resistant to infection with a murine cytomegalovirus strain lacking the dominant NK cell antigen m157, presumably as a consequence of perturbed NK cell maturation.68 Future studies will have to elucidate the reasons for the discrepancies between these NKG2D-deficient mouse models, which may be due to differences in the gene-targeting strategy or housing conditions.
The upregulation of NKG2DLs stimulates anticancer surveillance via NKG2D-dependent pathways that lead to NK- and T-cell activation. However, the prolonged expression of NKG2DLs induces the downregulation of NKG2D, in vitro and in vivo, hence promoting immune evasion, as documented in transgenic mice that constitutively and ubiquitously express MICA or Rae1.63,69 The ubiquitous expression of human MICA appeared to have no influence on lymphocyte subset distribution, but led to reduced levels of NKG2D on the surface of NK and activated CD8+ T cells. This resulted in impaired immune responses against MICA-overexpressing RMA cells as well as against Listeria-infected cells.62,63,69 Furthermore, a constitutive overexpression of Rae1ε in the normal epithelium caused both local and systemic defects in NKG2D-dependent NK cell cytotoxicity. Remarkably, in this model, cutaneous carcinogenesis correlated with the extent of NKG2D downregulation.62 Of note, transgenic mice genetically modified to express Rae1 in the dermal compartment under the control of a doxycycline-inducible promoter exhibit important changes in cellular infiltration during the early phase of local immune responses, resulting in multiple effects on carcinogenesis in the absence of additional pro-inflammatory stimuli.70 A more recent study using MICA-transgenic mice evaluated the function of NKG2D in the memory response of CD8+ T cells, demonstrating that NKG2D malfunction critically impairs the effector response of tumor-specific memory T cells to NKG2DL-expressing tumors.71
Whereas many cancer patients exhibit increased circulating levels of soluble NKG2DLs, which have been correlated in some studies with disease progression, the shedding of NKG2DLs from mouse cancer cells has not been investigated extensively apart from reports in Rae1-transgenic mice.36 To gain insights into the functional impact of soluble NKG2DLs in vivo, the mouse prostate cancer TRAMP-C2 cells overexpressing wild-type MICB, soluble MICB (rsMICB) or a shedding-resistant (non-cleavable) form of MICB (MICB.A2), were implanted in severe combined immunodeficient (SCID) mice. This study revealed that MIC shedding significantly contributes to oncogenesis, as mice receiving cancer cells expressing the shedding-resistant MICB variant did not develop any manifestations of prostate cancer.72 In conclusion, multiple studies in animal models confirmed that NKG2D acts as a central mediator of cancer immunosurveillance by promoting the cytotoxic functions of NK and T lymphocytes against malignant cells. This function is compromised by the persistent overexpression of NKG2DLs as well as by their shedding from transformed cells, resulting in decreased amounts of NKG2DLs, and NKG2D downregulation.62,72 These studies nurture the promise that immunotherapeutic interventions aimed at restoring the functionality of NKG2D combined with the targeted inhibition of NKG2DL shedding will be of benefit for cancer patients.
Relevance of the NKG2D/NKG2DL System for Immunotherapy
Malignant transformation induces the expression of NKG2DLs, as documented in a variety of human and mouse tumors including multiple types of leukemia, multiple myeloma, neuroblastoma, glioblastoma, as well as head and neck, hepatocellular, colorectal and cervical carcinoma.36,73 Presumably depending on the specific scenario, NKG2DLs appear to play quite different roles. As mentioned above, the expression of NKG2DLs on the surface of malignant cells enables their immunological clearance, thus preventing oncogenesis and tumor progression. In this respect, high levels of MICA were associated with a good prognosis among colorectal74 and breast carcinoma75 patients, while reduced NKG2DL expression reportedly correlates with early recurrence among hepatocellular carcinoma patients.76 Notably, the interactions of NKG2D with ULBP6 also appear to determine the clinical outcome of allogeneic stem cell transplantation, as a strong association between five distinct single nucleotide polymorphisms (SNPs) affecting the ULBP6-coding gene (RAET1L) and relapse-free survival has been reported.77
Conversely, NKG2DLs released from cancer cells by shedding, exosomal excretion or secretion are responsible for the escape of malignant cells from immune recognition, as they impair NKG2D function by blockade and internalization.4,54,73 Accumulating evidence indicates that the serum levels of soluble NKG2DLs increase with tumor progression.78 In this context, soluble ULBP2 has been proposed to constitute an indicator of poor prognosis in melanoma patients.79 Moreover, the accumulation of soluble NKG2DLs in the serum of neuroblastoma patients was associated with a drastic reduction in the cytotoxicity of infused donor NK cells upon haploidentical stem cell transplantation.80 Along these lines, Huang and colleagues suggested that screening cancer patients for the levels of MICA or MICB on the surface of transformed cells and the rate of MICA and MICB shedding might be useful to decide on treatment regimens boosting NKG2DL expression prior to the adoptive transfer of NKG2D-expressing cells.81
Strategies to overcome the escape of malignant cells from NKG2D-dependent immunosurveillance are of interest for the clinic and include the administration of substances such as HDACis, to upregulate MICA and/or MICB expression,44,46,47 as well as compounds that prevent the shedding of NKG2DLs from tumor cells, hence limiting the inhibition of NKG2D-dependent cytotoxicity in immune effector cells.44,73 For example, the HDACi valproic acid was reported to cause the upregulation of MICA, MICB and ULBP2 by an extracellular signal-regulated kinase (ERK)-dependent signaling cascade, increasing the NKG2D-dependent elimination of myeloma cells by NK cells.82 However, valproic acid has also been described to have negative effects on NK cell functions such as the downregulation of NKG2D and other activating receptors, resulting in the suppression of cytotoxicity and cytokine secretion.83,84 These data indicate that the pleiotropic effects of these compounds, which may vary with concentration and clinical setting, must be carefully considered for the development of immunotherapeutic regimens.
Recent data from a mouse model of breast carcinoma suggest that NKG2D reinforces the antitumor activity of antibodies targeting cytotoxic T lymphocyte-associated protein 4 (CTLA4) by suppressing the increased motility of tumor-infiltrating CD8+ T cells normally developing in this context.85 In this setting, the antitumor activity of CD8+ T cells was improved by combining anti-CTLA4 antibodies with ionizing radiation, promoting the expression of NKG2DLs on the surface of transformed cells. These data suggest that anti-CTLA4 immunotherapy which is currently approved by FDA for use in cancer patients86 can be complemented with strategies that enhance NKG2D function, including interventions that promote the expression of NKG2DLs on the surface of cancer cells, neutralize soluble MICA/MICB or prevent MICA/MICB shedding from tumor cells. The activation of NKG2D has also been reported to enhance the antitumor effects of various tumor-targeting antibodies.87,88 Furthermore, substantial antitumor effects have been reported for bispecific fusion proteins that are capable of eliciting NKG2D signaling.89,90 Similarly, the use of cytotoxic lymphocytes targeted to malignant cells with chimeric antigen receptors (CARs) that recognize tumor-associated NKG2DLs provides an appealing strategy for the development of novel immunotherapeutic anticancer interventions.91 Sentman and colleagues have shown that the adoptive transfer of T cells genetically modified to express a NKG2D-targeting CAR exert therapeutic effects in mice bearing various types of cancer.92,93 More recently, T cells have been successfully redirected to Ewing’s sarcoma cells with a chimeric NKG2D receptor.94 Still, the antitumor activity of these strategies critically depends on the robust expression of NKG2DL by malignant cells as well as on the aversion of tumor-derived factors that promote NKG2D downregulation.
Outlook
NKG2D is among the best characterized activating NK receptors with a major relevance for anticancer therapy. Although profound insights into the molecular events that regulate the expression of NKG2DLs on the surface of cancer cells have already been obtained, many questions remain open. For clinical purposes, it will be important to determine the heterogeneity of NKG2DL expression within a given tumor as well as the main factors that drive the expression of specific NKG2DLs and their release. Most studies employing small compounds to upregulate the expression of NKG2DL that have been performed so far relied on cultured cancer cells, and hence need to be validated in appropriate mouse models. Moreover, the role of soluble NKG2DLs in tumor progression has not yet been clearly defined. Therefore, it will be important to include the quantification of soluble NKG2DLs in the serum of large cohorts of cancer patients and healthy individuals, allowing for the assessment of the prognostic value of circulating NKG2DLs alone or in combination with established biomarkers. In conclusion, harnessing the natural capacity of cytotoxic lymphocytes to eliminate cancer cells in a NKG2D-dependent manner and exploiting the inducible NKG2DL expression by malignant cells offer attractive prospects for the development of novel immunotherapeutic anticancer regimens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The laboratories of E.U. and J.K. are supported by the LOEWE Center for Cell and Gene Therapy Frankfurt funded by Hessisches Ministerium für Wissenschaft und Kunst (HMWK) funding reference number III L 4- 518/17.004 (2010). E.U. is supported by grants of the Deutsche Krebshilfe e.V. and the Wilhelm-Sander Stiftung. J.K. received grants from the Wilhelm-Sander Stiftung, the German Federal Ministry of Health (BMG) and the Ministry of Higher Education, Research and the Arts of the State of Hessen (HMWK). The laboratory of A.C. is supported by grants by the Deutsche José Carreras Leukämie-Stiftung e.V. (DJCLS 11/06), by the Deutsche Krebshilfe e.V. and by the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and the Israel's Ministry of Science and Technology (MOST). The laboratory of A.S. is supported by grants by the Deutsche Krebshilfe e.V. and the German Research Council. The authors are grateful to Franziska Ganss for support in figure artwork.
Citation: Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. OncoImmunology 2013; 2:e26097;
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26097
References
- 1.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–91. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 3.Zafirova B, Wensveen FM, Gulin M, Polić B. Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci. 2011;68:3519–29. doi: 10.1007/s00018-011-0797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–58. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 6.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–43. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 7.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–80. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat Immunol. 2001;2:443–51. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–16. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–35. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 11.Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173:1017–20. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 13.Cerwenka A, Lanier LL. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens. 2003;61:335–43. doi: 10.1034/j.1399-0039.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 14.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 15.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–7. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–51. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 17.Benitez AC, Dai Z, Mann HH, Reeves RS, Margineantu DH, Gooley TA, Groh V, Spies T. Expression, signaling proficiency, and stimulatory function of the NKG2D lymphocyte receptor in human cancer cells. Proc Natl Acad Sci U S A. 2011;108:4081–6. doi: 10.1073/pnas.1018603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 19.Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci U S A. 2005;102:7641–6. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7:524–32. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 21.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–9. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 22.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–5. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 23.Zompi S, Hamerman JA, Ogasawara K, Schweighoffer E, Tybulewicz VL, Di Santo JP, Lanier LL, Colucci F. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–72. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 24.McFarland BJ, Kortemme T, Yu SF, Baker D, Strong RK. Symmetry recognizing asymmetry: analysis of the interactions between the C-type lectin-like immunoreceptor NKG2D and MHC class I-like ligands. Structure. 2003;11:411–22. doi: 10.1016/S0969-2126(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 25.McFarland BJ, Strong RK. Thermodynamic analysis of degenerate recognition by the NKG2D immunoreceptor: not induced fit but rigid adaptation. Immunity. 2003;19:803–12. doi: 10.1016/S1074-7613(03)00320-0. [DOI] [PubMed] [Google Scholar]
- 26.Steinle A, Groh V, Spies T. Diversification, expression, and gamma delta T cell recognition of evolutionarily distant members of the MIC family of major histocompatibility complex class I-related molecules. Proc Natl Acad Sci U S A. 1998;95:12510–5. doi: 10.1073/pnas.95.21.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinle A, Li P, Morris DL, Groh V, Lanier LL, Strong RK, Spies T. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53:279–87. doi: 10.1007/s002510100325. [DOI] [PubMed] [Google Scholar]
- 28.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–44. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Messina L, Reyburn HT, Valés-Gómez M. Human NKG2D-ligands: cell biology strategies to ensure immune recognition. Front Immunol. 2012;3:299. doi: 10.3389/fimmu.2012.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–7. doi: 10.1016/S1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 31.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 32.Schrambach S, Ardizzone M, Leymarie V, Sibilia J, Bahram S. In vivo expression pattern of MICA and MICB and its relevance to auto-immunity and cancer. PLoS One. 2007;2:e518. doi: 10.1371/journal.pone.0000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosman D, Müllberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 34.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:12445–50. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung H, Hsiung B, Pestal K, Procyk E, Raulet DH. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med. 2012;209:2409–22. doi: 10.1084/jem.20120565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–85. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern-Ginossar N, Mandelboim O. An integrated view of the regulation of NKG2D ligands. Immunology. 2009;128:1–6. doi: 10.1111/j.1365-2567.2009.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–11. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 40.Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011;71:5998–6009. doi: 10.1158/0008-5472.CAN-10-3211. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Lakshmikanth T, Garofalo C, Enge M, Spinnler C, Anichini A, Szekely L, Kärre K, Carbone E, Selivanova G. Pharmacological activation of p53 triggers anticancer innate immune response through induction of ULBP2. Cell Cycle. 2011;10:3346–58. doi: 10.4161/cc.10.19.17630. [DOI] [PubMed] [Google Scholar]
- 42.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasser S, Raulet D. The DNA damage response, immunity and cancer. Semin Cancer Biol. 2006;16:344–7. doi: 10.1016/j.semcancer.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Krieg S, Ullrich E. Novel immune modulators used in hematology: impact on NK cells. Front Immunol. 2012;3:388. doi: 10.3389/fimmu.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, Lauer UM, Bitzer M, Salih HR. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin Cancer Res. 2008;14:3520–8. doi: 10.1158/1078-0432.CCR-07-4744. [DOI] [PubMed] [Google Scholar]
- 46.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–9. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 47.Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–45. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 48.López-Soto A, Folgueras AR, Seto E, Gonzalez S. HDAC3 represses the expression of NKG2D ligands ULBPs in epithelial tumour cells: potential implications for the immunosurveillance of cancer. Oncogene. 2009;28:2370–82. doi: 10.1038/onc.2009.117. [DOI] [PubMed] [Google Scholar]
- 49.Fiegler N, Textor S, Arnold A, Rölle A, Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M, Cerwenka A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood. 2013;122:684–93. doi: 10.1182/blood-2013-02-482513. [DOI] [PubMed] [Google Scholar]
- 50.Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky N, Bar-Mag T, et al. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012;72:5463–72. doi: 10.1158/0008-5472.CAN-11-2671. [DOI] [PubMed] [Google Scholar]
- 51.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72:460–71. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 52.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 53.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 54.Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci. 2008;13:3448–56. doi: 10.2741/2939. [DOI] [PubMed] [Google Scholar]
- 55.Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–76. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 56.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 57.Jonjić S, Polić B, Krmpotić A. Viral inhibitors of NKG2D ligands: friends or foes of immune surveillance? Eur J Immunol. 2008;38:2952–6. doi: 10.1002/eji.200838823. [DOI] [PubMed] [Google Scholar]
- 58.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98:11521–6. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayakawa Y, Kelly JM, Westwood JA, Darcy PK, Diefenbach A, Raulet D, Smyth MJ. Cutting edge: tumor rejection mediated by NKG2D receptor-ligand interaction is dependent upon perforin. J Immunol. 2002;169:5377–81. doi: 10.4049/jimmunol.169.10.5377. [DOI] [PubMed] [Google Scholar]
- 62.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 63.Wiemann K, Mittrücker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–9. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 64.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–8. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayakawa Y, Smyth MJ. NKG2D and cytotoxic effector function in tumor immune surveillance. Semin Immunol. 2006;18:176–85. doi: 10.1016/j.smim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheppard S, Triulzi C, Ardolino M, Serna D, Zhang L, Raulet DH, Guerra N. Characterization of a novel NKG2D and NKp46 double-mutant mouse reveals subtle variations in the NK cell repertoire. Blood. 2013;121:5025–33. doi: 10.1182/blood-2012-12-471607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zafirova B, Mandarić S, Antulov R, Krmpotić A, Jonsson H, Yokoyama WM, Jonjić S, Polić B. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity. 2009;31:270–82. doi: 10.1016/j.immuni.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–7. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 70.Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–54. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 71.André MC, Sigurdardottir D, Kuttruff S, Pömmerl B, Handgretinger R, Rammensee HG, Steinle A. Impaired tumor rejection by memory CD8 T cells in mice with NKG2D dysfunction. Int J Cancer. 2012;131:1601–10. doi: 10.1002/ijc.26191. [DOI] [PubMed] [Google Scholar]
- 72.Wu JD, Atteridge CL, Wang X, Seya T, Plymate SR. Obstructing shedding of the immunostimulatory MHC class I chain-related gene B prevents tumor formation. Clin Cancer Res. 2009;15:632–40. doi: 10.1158/1078-0432.CCR-08-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groth A, Klöss S, von Strandmann EP, Koehl U, Koch J. Mechanisms of tumor and viral immune escape from natural killer cell-mediated surveillance. J Innate Immun. 2011;3:344–54. doi: 10.1159/000327014. [DOI] [PubMed] [Google Scholar]
- 74.Nicholas PK, Leuner JD, Hatfield JM, Corless IB, Marr KH, Mott MK, Cross-Skinner S. Using the Cancer Rehabilitation Questionnaire in patients with colorectal cancer. Rehabil Nurs. 2006;31:106–13. doi: 10.1002/j.2048-7940.2006.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 75.de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA, Jafferji I, Trowsdale J, Liefers GJ, van de Velde CJ, et al. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer. 2012;12:24. doi: 10.1186/1471-2407-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamimura H, Yamagiwa S, Tsuchiya A, Takamura M, Matsuda Y, Ohkoshi S, Inoue M, Wakai T, Shirai Y, Nomoto M, et al. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J Hepatol. 2012;56:381–8. doi: 10.1016/j.jhep.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 77.Antoun A, Vekaria D, Salama RA, Pratt G, Jobson S, Cook M, Briggs D, Moss P. The genotype of RAET1L (ULBP6), a ligand for human NKG2D (KLRK1), markedly influences the clinical outcome of allogeneic stem cell transplantation. Br J Haematol. 2012;159:589–98. doi: 10.1111/bjh.12072. [DOI] [PubMed] [Google Scholar]
- 78.Hilpert J, Grosse-Hovest L, Grünebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189:1360–71. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 79.Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, et al. Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res. 2009;15:5208–15. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- 80.Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader P, et al. IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol. 2010;40:3255–67. doi: 10.1002/eji.201040568. [DOI] [PubMed] [Google Scholar]
- 81.Huang B, Sikorski R, Sampath P, Thorne SH. Modulation of NKG2D-ligand cell surface expression enhances immune cell therapy of cancer. J Immunother. 2011;34:289–96. doi: 10.1097/CJI.0b013e31820e1b0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu X, Tao Y, Hou J, Meng X, Shi J. Valproic acid upregulates NKG2D ligand expression through an ERK-dependent mechanism and potentially enhances NK cell-mediated lysis of myeloma. Neoplasia. 2012;14:1178–89. doi: 10.1593/neo.121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogbomo H, Michaelis M, Kreuter J, Doerr HW, Cinatl J., Jr. Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett. 2007;581:1317–22. doi: 10.1016/j.febslet.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 84.Rossi LE, Avila DE, Spallanzani RG, Ziblat A, Fuertes MB, Lapyckyj L, Croci DO, Rabinovich GA, Domaica CI, Zwirner NW. Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. J Leukoc Biol. 2012;91:321–31. doi: 10.1189/jlb.0711339. [DOI] [PubMed] [Google Scholar]
- 85.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deguine J, Breart B, Lemaître F, Bousso P. Cutting edge: tumor-targeting antibodies enhance NKG2D-mediated NK cell cytotoxicity by stabilizing NK cell-tumor cell interactions. J Immunol. 2012;189:5493–7. doi: 10.4049/jimmunol.1202065. [DOI] [PubMed] [Google Scholar]
- 88.Inagaki A, Ishida T, Yano H, Ishii T, Kusumoto S, Ito A, Ri M, Mori F, Ding J, Komatsu H, et al. Expression of the ULBP ligands for NKG2D by B-NHL cells plays an important role in determining their susceptibility to rituximab-induced ADCC. Int J Cancer. 2009;125:212–21. doi: 10.1002/ijc.24351. [DOI] [PubMed] [Google Scholar]
- 89.von Strandmann EP, Hansen HP, Reiners KS, Schnell R, Borchmann P, Merkert S, Simhadri VR, Draube A, Reiser M, Purr I, et al. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood. 2006;107:1955–62. doi: 10.1182/blood-2005-05-2177. [DOI] [PubMed] [Google Scholar]
- 90.Kellner C, Hallack D, Glorius P, Staudinger M, Mohseni Nodehi S, de Weers M, van de Winkel JG, Parren PW, Stauch M, Valerius T, et al. Fusion proteins between ligands for NKG2D and CD20-directed single-chain variable fragments sensitize lymphoma cells for natural killer cell-mediated lysis and enhance antibody-dependent cellular cytotoxicity. Leukemia. 2012;26:830–4. doi: 10.1038/leu.2011.288. [DOI] [PubMed] [Google Scholar]
- 91.Müller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, Tonn T, Wels WS. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411–23. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–51. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spear P, Barber A, Rynda-Apple A, Sentman CL. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol. 2013;91:435–40. doi: 10.1038/icb.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lehner M, Götz G, Proff J, Schaft N, Dörrie J, Full F, Ensser A, Muller YA, Cerwenka A, Abken H, et al. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]