Abstract
The levels of regenerating islet-derived 3β (REG3β) in the serum and pancreatic juice of patients affected by pancreatic ductal adenocarcinomas are increased. However, whether such an elevation is relevant to oncogenesis and tumor progression has not yet been carefully examined. We have recently demonstrated that silencing REG3β in a pancreatic cancer model impairs tumor growth by skewing macrophage polarization.
Keywords: REG3β, macrophage polarization, pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) continues to be a leading cause of cancer-related deaths worldwide, mostly due to its problematic diagnosis and high resistance to standard therapeutic regimens. During the past decade, numerous agents that target specific properties of malignant cells, including their increased proliferative potential, their capacity to stimulate angiogenesis and their metastatic attitude, have been assessed, alone or in combination with gemcitabine, as potential regimens for the treatment of PDAC. Unfortunately, most of these approaches have failed so far to significantly increase the overall survival of PDAC patients.1 Thus, there is an urgent need for novel regiments that provide true clinical benefits to subjects with PDAC.
As it stands, the biology of developing tumors cannot be simply reconducted to that of malignant cells: remarkable progress has been made in the understanding of the functional contributions made by stromal cells in this context. The mutual interactions between neoplastic and stromal cells are nowadays recognized as fundamental aspects of tumor growth, invasion, and metastasis. One of the defining features of PDAC is the generation of a dense desmoplastic stroma, which can account for up to 80% of the tumor mass.2 This compartment contains a significant amount of macrophages, which are present in early PDAC lesions and persist throughout tumor progression.3
Macrophages are recruited to the tumor microenvironment in the course of tumor progression, hence switching into tumor-associated macrophages (TAMs). TAMs resemble alternatively activated M2 macrophages in that they both stimulate tumor growth and angiogenesis while suppressing the elicitation of adaptive immune responses.4 In other setting, macrophages can acquire a classical M1 phenotype, which is characterized by the secretion of pro-inflammatory cytokines and by a robust cytotoxic activity against pathogens and malignant cells. Because of the elevated plasticity of macrophages, the M1 and M2 phenotypes are likely to represent the opposite ends of a continuous functional spectrum.
Studies aimed at characterizing the immune infiltrate of PDAC lesions have detected high levels of M2 markers in TAMs from PDAC patients as well as from a murine model of metastatic pancreatic adenocarcinoma. Interestingly, these M2-polarized macrophages were associated with increased disease burden and poor prognosis due to accelerated lymphatic metastasis.5
Regenerating islet-derived 3β (REG3β) is a soluble protein rarely secreted by the normal pancreas, but highly produced by injured pancreatic tissues. Our group has previously characterized the anti-inflammatory functions of REG3β in the course of pancreatic inflammation, a potential driver of pancreatic carcinogenesis. Hence, the targeted disruption of Reg3b in mice resulted in enhanced inflammation of the pancreas.6 Increased levels of REG3β have also been detected in the serum and pancreatic juice from PDAC patients, but the function of this protein in pancreatic carcinogenesis has not yet been carefully examined.
In a recent issue of Cancer Research,7 we demonstrated that REG3β significantly contributes to the establishment of an immunosuppressive microenvironment during pancreatic cancer progression. The deletion of Reg3b drastically impaired the growth of pancreatic neoplasms in mice, correlating with decreased angiogenesis and increased apoptosis of cancer cells. Moreover, REG3β not only inhibited the acquisition of an antitumor M1 phenotype by macrophages exposed to pro-inflammatory stimuli in vitro, but also promoted their skewing toward an M2 polarization by favoring the expression of M2-associated genes.
The analysis of angiogenesis-related factors produced by macrophages obtained from tumor-bearing Reg3b−/− mice revealed marked reductions in matrix metallopeptidase 9 (MMP9) and vascular endothelial growth factor (VEGF), which is known to recruit macrophages to the tumor microenvironment. These findings provided an explanation for the poor vascularization and abrogated metastatic potential of pancreatic tumors developing in Reg3b−/− mice, suggesting that REG3β critically contributes to the angiogenic switch that is required for the progression of pancreatic neoplasms.
Since we observed the same extent of macrophage infiltration in tumors growing in wild-type and Reg3b−/− mice, we hypothesized that REG3β would act by skewing TAMs toward an immunosuppressive and pro-tumorigenic M2 phenotype. We confirmed this hypothesis and we demonstrated that the increased apoptotic demise of cancer cells growing in Reg3b−/− mice is not a direct consequence of the anti-apoptotic role attributed to REG3β but rather originate from an indirect effect mediated by polarized macrophages.
Accumulating evidence indicates pharmacological skewing macrophage polarization from an M2 to an M1 phenotype may result in some degree of antitumor activity. In a study based on different tumor models, Guiducci et al.8 tested whether the treatment of tumor-bearing mice with a macrophage chemoattractant would synergize with an intervention to switch the macrophage phenotype from M2 to M1. Indeed, the macrophage chemoattractant given in combination with a microbial stimulus and a monoclonal antibody specific for the interleukin-10 receptor promptly skewed the phenotype of TAMs and triggered an innate immune response that mediated the regression of large established tumors.
The importance of the polarization state of TAMs has also been highlighted in murine models of PDAC as well as in PDAC patients. For instance, an agonist antibody targeting the co-stimulatory molecule CD40 has recently been shown to exert antineoplastic effects in a cohort of metastatic PDAC patients.9 In this setting, the activation of CD40 switched M2-like TAMs into antitumor M1-like TAMs. These macrophages rapidly infiltrated pancreatic lesions, became tumoricidal and depleted the tumor stroma, thus restoring immunosurveillance.
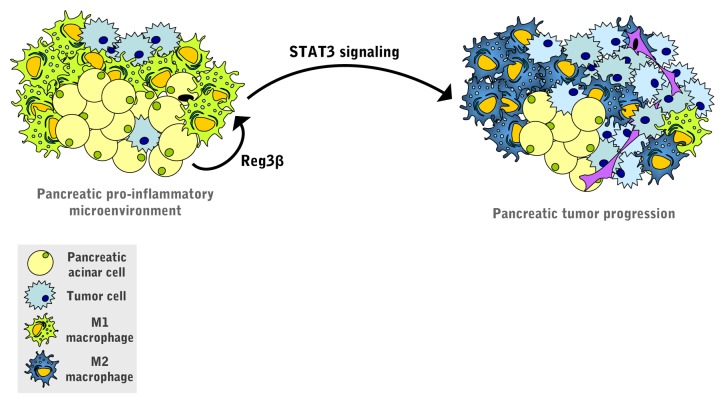
Finally, mechanistic studies based on primary macrophages revealed that the REG3β-induced switch toward the M2 phenotype is associated with the activation of the transcription factor signal transducer and activator of transcription 3 (STAT3) (Fig. 1). STAT3 is aberrantly activated in human PDACs and promotes the progression of pancreatic intraepithelial neoplasia by transactivating anti-apoptotic and pro-proliferative genes.10
Figure 1. Pro-tumorigenic role of REG3β in pancreatic adenocarcinoma. Regenerating islet-derived 3β (REG3β) secreted by the acinar cells that surround pancreatic tumors acts in a paracrine manner on peri-tumoral macrophages, hence skewing them toward the M2 phenotype. REG3β operates by activating signal transducer and activator of transcription 3 (STAT3), a transcription factor that is required for the development and progression of pancreatic cancer.
With this in mind and considering the central role of REG3β in pancreatic disorders, the inhibition of REG3β—intended to reprogram TAMs toward an antitumor phenotype—stands out as a promising and effective approach for the treatment of pancreatic cancer.
Disclosure of Potential Conflicts of Interest
There are no conflicts of interest to disclose.
Glossary
Abbreviations:
- PDAC
pancreatic ductal adenocarcinoma
- REG3β
regenerating islet-derived 3β
- STAT3
signal transducer and activator of transcription 3
- TAM
tumor-associated macrophage
Citation: Folch-Puy E. Reg3β contributes to the immunosuppressive tumor microenvironment in pancreatic cancer. OncoImmunology 2013; 2:e26404; 10.4161/onci.26404
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26404
References
- 1.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy. 2011;3:517–37. doi: 10.2217/imt.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 3.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–67. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 4.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson DD, Meng X, Fullerton DA, Moore EE, Lee JH, Ao L, Silliman CC, Barnett CC., Jr. Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1067–75. doi: 10.1152/ajpregu.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gironella M, Folch-Puy E, LeGoffic A, Garcia S, Christa L, Smith A, Tebar L, Hunt SP, Bayne R, Smith AJ, et al. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut. 2007;56:1091–7. doi: 10.1136/gut.2006.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gironella M, Calvo C, Fernández A, Closa D, Iovanna JL, Roselló-Catafau J, Folch-Puy E. Reg3β deficiency impairs pancreatic tumor growth by skewing macrophage polarization. Cancer Res. 2013;73:5682–94. doi: 10.1158/0008-5472.CAN-12-3057. [DOI] [PubMed] [Google Scholar]
- 8.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–46. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 9.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wörmann SM, Diakopoulos KN, Lesina M, Algül H. The immune network in pancreatic cancer development and progression. Oncogene. 2013 doi: 10.1038/onc.2013.257. Forthcoming. [DOI] [PubMed] [Google Scholar]