Abstract
Survivin-3B (S-3B), an alternative splice isoform of survivin, plays a key role in tumorigenesis. S-3B promotes the escape of malignant cells from immune recognition by blocking the cytotoxicity of natural killer (NK) cells. Such an effect reflects the ability of S-3B to interfere with the assembly of the so-called “death-inducing signaling complex” upon the interaction of FAS with its ligand (FASL). S-3B also inhibits the activation of caspase-6, thus increasing the resistance of neoplastic cells to granzyme B and various chemotherapeutics.
Keywords: caspase-6, caspase-8, survivin-3B, tumorigenesis, tumor escape, treatment response
Tumors develop along a cascade of steps that include (as an early event) the dysregulation of alternative splicing. Such an aberrant splicing results in the synthesis of new polypeptides, some of which are cancer-specific. One example of these cancer-specific proteins is survivin-3B (S-3B), which originates as a splicing variant of survivin (baculoviral IAP repeat containing 5, BIRC5).1 Conversely, survivin and 3 other alternative isoforms, namely, survivin-ΔEx3, survivin-2B, and survivin-2α are expressed in both neoplastic and normal cells. It is therefore important to understand whether S-3B plays a role in oncogenesis and tumor progression.
The S-3B-coding mRNA includes the 4 first exons of BIRC5 plus 69 nucleotides from intron 3, a new coding sequence (named exon 3B) that contains a stop codon.2 Similar to survivin (but not other survivin isoforms) S-3B harbors a complete BIR domain. Thus, S-3B mediates anti-apoptotic functions and has been involved in the resistance of malignant cells to cisplatin3 and 5-fluorouracil.4
The cancer-specific expression profile of S-3B prompted us to focus on its effects on tumorigenesis. Normally, the immune system is programmed to eliminate abnormal cells that may become malignant. The elimination of such pre-cancerous cells is mainly attributable to CD8+ T lymphocytes and natural killer (NK) cells. Only when neoplastic cells resist the cytotoxic activity of immune effectors, they can proliferate unrestrained and generate tumors, a process that is known as immune escape. In the course of tumor progression, immune cells of multiple types infiltrate malignant lesions. Some of these cells exert tumor-supporting functions, while others—including NK cells—mediate robust antitumor effects.5
To get insights into the functions of S-3B, we first tested the effect of its overexpression on a non-tumorigenic cell line. We observed that inoculation of originally non-tumorigenic cells that had been engineered to overexpress S-3B in nude mice, which have no T lymphocytes but harbor high NK-cell activity, promoted the development of malignant lesions. We therefore hypothesized that S-3B could inhibit the antitumor activity of NK cells. To test this hypothesis, we downregulated S-3B in vivo by injecting specific small-interfering RNAs (siRNAs) into neoplastic lesions generated by a highly tumorigenic cell line, an intervention that considerably reduced tumor growth. The impact of S-3B on the cytotoxic activity of NK cells was confirmed as the effects of S-3B-targeting siRNAs completely disappeared in NK cell-depleted nude mice. In vitro, S-3B was shown to interact with pro-caspase-8, hence preventing its proteolytic maturation upon the interaction of FAS with its ligand (FASL). Owing to the ability to inhibit the formation of the so-called death-inducing signaling complex (DISC), S-3B completely blocked the ability of NK cells to provoke the apoptotic demise of cancer cells. NK cells can also eliminate unwanted cells via the granzyme B/perforin system. This mechanism operates through mitochondrial depolarization, cytochrome c release and the activation of caspases-9, -3, -6 and -7. Since intrinsic apoptosis is also the cell death subroutine whereby most anticancer agents exert their activity, we decided to explore the effects of S-3B on the response of neoplastic cells to staurosporine and 5-fluorouracil. Upon exposure to these treatments, cells expressing high levels of S-3B not only failed to die, but also were able to divide and form colonies in clonogenic assays. In contrast, cells lacking S-3B died massively in response to apoptotic stimuli. By studying the mechanisms underlying intrinsic apoptosis as triggered in our model by staurosporine and 5-fluorouracil, we observed that cancer cells underwent mitochondrial depolarization followed by the activation of caspases-9 and -3 irrespective of S-3B expression levels. Rather, S-3B was involved in the events downstream of caspase-3 activation, notably as it inhibited the cleavage and activation of pro-caspase-6. Thus, S-3B sequestered pro-caspase-6 upon physical interaction, thus impeding its activation by active caspase-3.
Taken together, these data suggest that combining S-3B-targeting interventions with chemotherapy could result in a superior efficacy by improving both the activity of immune cells and the direct cytotoxicity of antineoplastic agents. In support of this hypothesis, we demonstrated that the association of S-3B-depleting siRNAs and 5-fluorouracil improves anticancer responses in mice.
As S-3B appeared to provide cancer cells with improved protection as compared with survivin, through different mechanisms, we studied in detail the sequence of S-3B. S-3B shares the first 113 amino acids with survivin but contains 7 amino acids at the C-terminus that differ from the corresponding residues of survivin. We showed that this short polypeptide, which we named LEO sequence, is required for the interaction of S-3B with its targets.
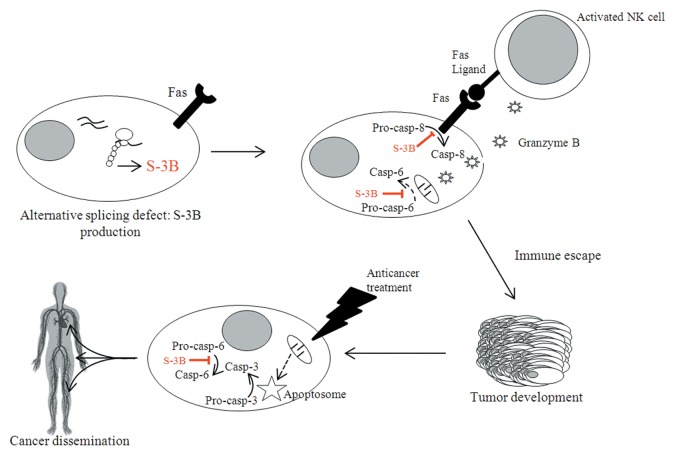
In conclusion, S-3B turned out to play a critical role in cancer initiation, progression and dissemination (Fig. 1). This protein is not the sole responsible for oncogenesis, but some evidence indicates that the expression level of S-3B might be related to tumor progression1 and poor disease outcome.6,7 The importance of S-3B in the resistance of cancer cells to immune effectors (especially NK cells) and chemotherapy, coupled to its tumor-specific expression pattern, should encourage the development of S-3B-targeting therapies, for example siRNA-based approaches or inhibitors of the LEO domain.
Figure 1. Involvement of survivin-3B in cancer initiation, progression, and dissemination. The defects in alternative splicing that normally accompany oncogenesis can result in the production of survivin-3B (S-3B). Pre-malignant cells are normally detected by the immune system, in particular by natural killer (NK) cells. Upon such a recognition, activated NK cells attempt to eliminate target cells by triggering FAS-dependent cell death and by secreting granzyme B. However, S-3B inhibits both the extrinsic and the intrinsic pathways of apoptosis. In this way, pre-malignant cells can escape elimination by the immune system and generate neoplastic lesions. In addition, S-3B inhibits the apoptotic response of cancer cells to chemotherapy and perhaps favors their metastatic dissemination. Dashed lines summarize several events occurring upstream of the represented process.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- BIR
baculovirus IAP repeat
- DISC
death-inducing signaling complex
- NK
natural killer
- S-3B
survivin-3B
Citation: Végran F, Boidot R. Survivin-3B promotes chemoresistance and immune escape by inhibiting caspase-8 and -6 in cancer cells OncoImmunology 2013; 2:e26328; 10.4161/onci.26328
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26328
References
- 1.Végran F, Mary R, Gibeaud A, Mirjolet C, Collin B, Oudot A, Charon-Barra C, Arnould L, Lizard-Nacol S, Boidot R. Survivin-3B potentiates immune escape in cancer but also inhibits the toxicity of cancer chemotherapy. doi: 10.1158/0008-5472.CAN-13-0036. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 2.Badran A, Yoshida A, Ishikawa K, Goi T, Yamaguchi A, Ueda T, Inuzuka M. Identification of a novel splice variant of the human anti-apoptopsis gene survivin. Biochem Biophys Res Commun. 2004;314:902–7. doi: 10.1016/j.bbrc.2003.12.178. [DOI] [PubMed] [Google Scholar]
- 3.Knauer SK, Bier C, Schlag P, Fritzmann J, Dietmaier W, Rödel F, Klein-Hitpass L, Kovács AF, Döring C, Hansmann ML, et al. The survivin isoform survivin-3B is cytoprotective and can function as a chromosomal passenger complex protein. Cell Cycle. 2007;6:1502–9. doi: 10.4161/cc.6.12.4305. [DOI] [PubMed] [Google Scholar]
- 4.Sawai K, Goi T, Hirono Y, Katayama K, Yamaguchi A. Survivin-3B gene decreases the invasion-inhibitory effect of colon cancer cells with 5-fluorouracil. Oncol Res. 2010;18:541–7. doi: 10.3727/096504010X12767359113848. [DOI] [PubMed] [Google Scholar]
- 5.Fregni G, Perier A, Avril MF, Caignard A. NK cells sense tumors, course of disease and treatments: Consequences for NK-based therapies. Oncoimmunology. 2012;1:38–47. doi: 10.4161/onci.1.1.18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boidot R, Végran F, Lizard-Nacol S. Predictive value of survivin alternative transcript expression in locally advanced breast cancer patients treated with neoadjuvant chemotherapy. Int J Mol Med. 2009;23:285–91. [PubMed] [Google Scholar]
- 7.Végran F, Boidot R, Bonnetain F, Cadouot M, Chevrier S, Lizard-Nacol S. Apoptosis gene signature of Survivin and its splice variant expression in breast carcinoma. Endocr Relat Cancer. 2011;18:783–92. doi: 10.1530/ERC-11-0105. [DOI] [PubMed] [Google Scholar]