Abstract
Nontypeable Haemophilus influenzae (NTHi) is an opportunistic pathogen which causes a variety of respiratory infections. The objectives of the study were to determine its antimicrobial susceptibility, to characterize the β-lactam resistance, and to establish a genetic characterization of NTHi isolates. Ninety-five NTHi isolates were analyzed by pulsed field gel electrophoresis (PFGE) and multi locus sequence typing (MLST). Antimicrobial susceptibility was determined by microdilution, and the ftsI gene (encoding penicillin-binding protein 3, PBP3) was PCR amplified and sequenced. Thirty (31.6%) isolates were non-susceptible to ampicillin (MIC≥2 mg/L), with 10 of them producing β-lactamase type TEM-1 as a resistance mechanism. After ftsI sequencing, 39 (41.1%) isolates showed amino acid substitutions in PBP3, with Asn526→ Lys being the most common (69.2%). Eighty-four patients were successfully treated with amoxicillin/clavulanic acid, ceftriaxone and levofloxacin. Eight patients died due either to aspiration or complication of their comorbidities. In conclusion, NTHi causing CAP in adults shows high genetic diversity and is associated with a high rate of reduced susceptibility to ampicillin due to alterations in PBP3. The analysis of treatment and outcomes demonstrated that NTHi strains with mutations in the ftsI gene could be successfully treated with ceftriaxone or fluoroquinolones.
Introduction
Haemophilus influenzae is a human-restricted pathogen which forms part of the normal nasopharyngeal microbiota [1]–[4]. This bacterial species is commonly divided into two different groups depending on the presence or absence of the polysaccharide capsule, with six serotypes (a–f) currently described in the encapsulated group. In children, serotype b (Hib) is responsible for most invasive diseases, although incidence has dramatically decreased since vaccine introduction [4]. Non-capsulated H. influenzae, also known as nontypeable H. influenzae (NTHi), colonizes asymptomatically the nasopharynx in healthy people, and is also a frequent cause of otitis media, sinusitis, conjunctivitis, community-acquired pneumonia (CAP) and exacerbations in chronic obstructive pulmonary disease (COPD) [1]–[4].
CAP is a common respiratory infection which frequently requires patient hospitalization. Current studies identify H. influenzae as either the second most common pathogen causing CAP, after Streptococcus pneumoniae [5], [6], or the third most common pathogen after S. pneumoniae and Mycoplasma pneumoniae [7]. In our geographical area, H. influenzae has been identified as the aetiological agent in 6–10% of CAP [8].
Aminopenicillin antibiotics have been used in the treatment of H. influenzae infections, and as a result, mechanisms of resistance against this group of antimicrobials have developed [9]–[11]. The most common mechanism of β-lactam resistance involves the production of a β-lactamase enzyme, usually TEM-1 type or, more rarely, ROB-1 type [12]. Alterations in penicillin-binding proteins (PBP3) have also been reported in different H. influenzae strains [13], [14]. This phenotype, also known as β-lactamase negative ampicillin resistance (BLNAR), is related to mutations in the ftsI gene (encoding the transpeptidase domain of PBP3) [15]. The frequency of resistance to other antimicrobials such as quinolones or azithromycin is, however, low [11], [16].
Epidemiological studies of individual patient groups are important for determining the level and mechanisms of antimicrobial resistance. In line with this goal, the present study had three main objectives: to determine the antimicrobial susceptibility of nontypeable H. influenzae strains isolated from patients with non-bacteremic CAP, to characterize the β-lactam resistance and to establish the clonal relatedness among these strains.
Materials and Methods
Ethics Statement
This work was approved by the ‘Comité Ètic d'Investigació Clínica del Hospital Universitari de Bellvitge’ and the written or oral informed consent was considered not necessary, because the source of bacterial isolates was anonymized and the study was retrospective.
Hospital Setting and Bacterial Strains
This study was carried out at the Hospital de Bellvitge in Barcelona, a hospital for adults serving a population of ca. 600,000 people. A retrospective review of computerized medical charts was performed in all patients seen at the hospital during the study period in order to record those with CAP criteria. Pneumonia was considered when a new infiltrate on a chest radiograph plus one or more of the following symptoms were detected: fever or hypothermia, new cough, pleuritic chest pain, dyspnea or altered breath sounds on auscultation [8]. Overall mortality was defined as death within 30 days of pneumonia diagnosis. Patients were considered cured when clinical findings of pneumonia had disappeared and there was radiological improvement.
A total of 95 NTHi isolates were collected from sputum samples of 92 patients diagnosed with non-bacteremic CAP between 2000 and 2009.
Only H. influenzae isolates from good quality sputum samples (<10 squamous cells and >25 leukocytes per low-power field) and with a predominance of Gram negative coccobacilli forms were considered [17].
Isolates were identified by conventional methodology and preserved by cryopreservation. Additionally, all isolates were identified by mass spectrometry using a MALDI-Biotyper version 3.0 (Bruker), following the manufacturer's recommendations. Differentiation between H. influenzae and H. haemolyticus was performed by the detection of fucK, iga and lgtC genes using a previously described methodology [18]. Isolates were identified as H. influenzae if they were positive for the three tested genes.
Biotyping, Serotyping and Antimicrobial Susceptibility
Biotypes were determined using three biochemical reactions: urease, indol and ornithine decarboxylase [19]. Serotyping was achieved with the latex agglutination Phadebact® Haemophilus Test (Bactus AB, Huddinge, Sweden) and by PCR as stipulated by Falla et al. [20]. Antimicrobial susceptibility was determined by microdilution according to the criteria of the Clinical Laboratory Standards Institute (CLSI) [21], [22]. β-lactamase production was screened using the chromogenic cephalosporin method (nitrocefin disks, BD, Madrid, Spain).
PCR and DNA Sequencing
Identification of β-lactamase type was performed by PCR on all the β-lactamase positive isolates using primers and conditions described previously [23]. For molecular characterization of PBP3, an internal region of the ftsI gene (796–1741 pb) was amplified by PCR and sequenced using previously described methodology [24].
Genotype Definition for Ampicillin Resistance
According to previous descriptions [25], [26] and on the basis of β-lactamase production and changes in the ftsI gene, H. influenzae isolates were classified into four genotypes: β-lactamase negative ampicillin susceptible (gBLNAS), strains without a detectable resistance mechanism; β-lactamase negative ampicillin resistant (gBLNAR), strains that did not produce a β-lactamase enzyme but which presented mutations in the transpeptidase domain of the ftsI gene; β-lactamase positive ampicillin resistant (gBLPAR), strains producing β-lactamase but which did not present mutations in ftsI; and β-lactamase positive amoxicillin/clavulanic acid resistant (gBLPACR), strains which presented both resistance mechanisms (β-lactamase production and mutations in the ftsI gene).
Molecular Typing
Pulsed field gel electrophoresis (PFGE)
Strain relatedness was determined by PFGE with the restriction enzyme SmaI (New England BioLabs, Ipswich, MA, USA), as instructed by the manufacturer. Molecular typing was performed on bacterial suspensions of H. influenzae grown on chocolate agar plates, as described by Dabernat et al. [24] but with some modifications. Briefly, bacterial suspensions were prepared in PIV (10 mM Tris-HCl [pH 8], 1 M NaCl) and adjusted to the same final concentration. The bacterial suspension was mixed with an equal volume of melted 1.5% low-melting point agarose (Life Technologies, Madrid, Spain) in order to prepare DNA-agarose plugs with a volume of 20 µl each. These were incubated for 5 h at 37°C in 1 ml of ST buffer (6 mM Tris-HCl [pH 8]; 1 M NaCl; 0.1 M EDTA [pH 8]) containing 0.5% Brij-58, 100 µg/mL lysozyme and 50 µg/ml RNAse. The agarose plugs were transferred into ES buffer (1 M EDTA, 1% sarcosyl) with 1 mg/mL proteinase K (Sigma Aldrich, Madrid, Spain) and incubated over night at 50°C. Finally, the plugs were rinsed three times at room temperature with TE buffer (10 mM Tris-HCl [pH 8]; 1 mM EDTA [pH 8]).
The DNA-embedded plugs were digested with 5 U of SmaI for 18 h at 25°C. DNA fragments were then separated in a 1% agarose gel (Megabase, BioRad) with 0.5% TBE buffer (45 mM Tris-base, 45 mM boric acid, 1.0 mM EDTA pH 8.0) in a contour-clamped homogenous electric field system (CHEF DR III; BioRad). The gels were run for 19 h at 14°C, using a constant voltage of 6 V/cm with an angle of 120° and an increasing pulse time from 1 s to 30 s. A bacteriophage λ, low-range PFG marker (New England BioLabs, Ipswich, MA, USA) was used as a size standard.
PFGE band patterns were analyzed using the Fingerprinting II Software 3.0 (BioRad). The similarity of the PFGE banding patterns was estimated with the Dice coefficient, setting the optimization and tolerance at 1%. Isolates with ≥80% relatedness were considered highly genetically related [27].
Multilocus sequence type (MLST)
Clinical isolates were analyzed by MLST in order to identify strain relatedness [28]. Allele number and sequence types (ST) were assigned using the H. influenzae MLST website (http://haemophilus.mlst.net). The overall database was analyzed using e-BURST v3 in order to define groups available on the H. influenzae MLST website.
Results
Patient Characteristics and Antimicrobial Susceptibility
NTHi isolates were recovered from 95 episodes of CAP in 92 patients. Sixty-four patients (69.6%) were men and the mean age was 68.15 years (SD±14.39). Comorbid conditions were present in 97% of patients, with COPD being the most frequent underlying disease (28.3%), followed by chronic heart disease (18.5%), malignancy (15.2%), diabetes mellitus (13%) and chronic renal failure (4.3%). Finally, 59.7% of patients were either current (13%) or past (46.7%) smokers.
Table 1 summarizes the antibiotic susceptibility of the NTHi isolates. All of them were susceptible to ceftriaxone, cefotaxime and levofloxacin. By contrast, 10.5% of the isolates were resistant to ampicillin due to the expression of a TEM-1 β-lactamase, and 23.2% presented intermediate resistance. The rate of resistance to cotrimoxazole was high (32.6%), whereas the frequency of resistance to amoxicillin/clavulanic acid, cefuroxime, tetracycline, chloramphenicol and azithromycin was low (<4%).
Table 1. Minimal inhibitory concentrations (MIC) of 10 antimicrobials. MIC against 95 NTHi isolates using the microdilution method according to CLSI breakpoints.
| Antimicrobials | MIC50 | MIC90 | Range | CLSIa | |
| (mg/L) | (mg/L) | %I | %R | ||
| Ampicillin | 0.5 | 2 | ≤0.25–≥16 | 23.2 | 10.5 |
| Amoxicillin/clavulanic acid b | 1 | 4 | ≤0.5–8 | 0 | 2.1 |
| Ceftriaxone | <0.06 | <0.06 | ≤0.06–0.12 | 0 | 0 |
| Cefotaxime | <0.06 | <0.06 | ≤0,06–0.12 | 0 | 0 |
| Cefuroxime | 2 | 4 | ≤0.5–≥8 | 3.1 | 1.1 |
| Tetracycline | ≤2 | ≤2 | ≤2–≥4 | 0 | 2.1 |
| Chloramphenicol | ≤2 | ≤2 | ≤2–8 | 0 | 1.1 |
| Azithromycin | 2 | 2 | ≤0.5–≥4 | 0 | 1.1 |
| Levofloxacin | ≤0.5 | ≤0.5 | ≤0.5–1 | 0 | 0 |
| Cotrimoxazole c | ≤0.5 | >2 | ≤0.5–≥2 | 0 | 32.6 |
a CLSI: Clinical and Laboratory Standards Institute. I: intermediate; R: resistant.
∶1.b The ratio of amoxicillin/clavulanic acid was 2
∶19.c The ratio of cotrimoxazole was 1
Mutation Patterns in the ftsI Gene
The sequence of ftsI encoding the transpeptidase region of PBP3 was determined in all the isolates. Table 2 summarizes the amino acid changes observed, corresponding to 41.1% of the isolates. The most common amino acid substitution was Asn526→ Lys (27/39, 69.2%), followed by Arg517→ His (2/39, 5.1%). The patterns observed were classified into groups I and II according to the criteria of Dabernat et al. [24].
Table 2. Amino acid substitutions in the transpeptidase domain of PBP3 identified in 95 NTHi isolates.
| Groupa | Amino acid substitutions | MIC (mg/L)b | BLc | No isolates | Sequence Type (ST) | |||||||||||
| Asp 350 | Ala 368 | Met 377 | Met 391 | Ala 545 | Gly 490 | Ala 502 | Arg 517 | Asn 526 | Ala 530 | Thr 532 | AMP | AMC | ||||
| I | His | 0.5–2 | 1–2 | - | 2 | 159 (n = 2) | ||||||||||
| IIa | Glu | Lys | Ser | 2 | 4 | - | 1 | 14 | ||||||||
| Lys | Ser | 2 | 4 | - | 2 | 142, 414 | ||||||||||
| Lys | 2 | 4 | - | 1 | 998 | |||||||||||
| Asn | Glu | Lys | Ser | 1 | 1 | - | 1 | 201 | ||||||||
| IIb | Asn | Ile | Val | Lys | ≥16 | 8 | + | 1 | 165 | |||||||
| Asn | Ile | Val | Lys | 1–2 | 4 | - | 3 | 14, 142, 367 | ||||||||
| Asn | Ile | Glu | Val | Lys | 1–2 | 1–2 | - | 3 | 204, 556, 1177 | |||||||
| IIc | Asn | Thr | Lys | ≥16 | 8 | + | 1 | 1171 | ||||||||
| Thr | Lys | 2 | 2–4 | - | 8 | 1048, 993, 819 (n = 2), 1162, 996, 1000, 409 | ||||||||||
| Asn | Thr | Lys | 1–2 | 1–4 | - | 6 | 556, 648, 1171, 999, 159 (n = 2) | |||||||||
| Miscellaneous | Asn | ≥16 | 4 | + | 1 | 997 | ||||||||||
| Thr | ≤0.5 | 1 | - | 2 | 267, 1163 | |||||||||||
| Asn | ≤0.5 | ≤0.5–1 | - | 2 | 388, 1143 | |||||||||||
| Ile | 0.5 | 1 | - | 1 | 994 | |||||||||||
| Val | 0.5 | 1 | - | 1 | 85 | |||||||||||
| Asn | Asn | 0.5 | 1 | - | 1 | 425 | ||||||||||
| Val | ≤0.25 | ≤0.5 | - | 2 | 991 (n = 2) | |||||||||||
| No changes | 8–≥16 | 1–4 | + | 7 | 57, 142, 160, 270, 272, 836, 1172 | |||||||||||
| ≤0.25–1 | ≤0.5–2 | - | 49 | d | ||||||||||||
[24]; the miscellaneous group was classified according to the criteria of García-Cobos et al. [10] and the data from this study.a The isolates were classified into groups I, IIa, IIb and IIc, according to the criteria of Dabernat et al.
≤1 mg/L; AMC Resistant: ≥8/4 mg/L; AMC susceptible: ≤4/2 mg/L;b AMP Resistant: >4 mg/L; AMP Intermediate: 2 mg/L; AMP Susceptible
+: positive; -: negative);c BL: Beta-lactamase production (
= 3), ST36, ST98, ST103, ST139 (n = 2), ST145 (n = 3), ST159 (n = 3), ST183, ST203 (n = 3), ST241 (n = 2), ST245, ST266, ST270, ST272, ST385, ST408, ST414 (n = 2), ST519 (n = 4), ST582, ST679, ST714, ST974, ST989, ST990, ST992, ST995, ST1174, ST1176, ST1178, ST1179, ST1180, ST1181, ST1182, ST1183 and ST1184.d ST11 (n
Two isolates were classified as Group I and presented the Arg517→ His substitution alone. Group II included 27 isolates subdivided into three subgroups: i) 5 isolates belonged to the subgroup IIa (1 isolate with Asn526→ Lys, and the remaining 4 isolates with other mutations); ii) 7 isolates were classified as subgroup IIb, defined by Asn526→ Lys and Ala502→ Val substitutions (those isolates also presented the substitutions Asp350→ Asn and Met377→ Ile, and one of them also had a Gly490→ Glu); iii) the subgroup IIc, characterized by Asn526→ Lys and Ala502→ Thr substitutions, was the most common, with 15 isolates. No isolates were observed in subgroup IId or in groups III and III-like (previously described by García-Cobos et al. [10]).
Six patterns (10 isolates) were characterized and classified into the miscellaneous group: four of them (6 isolates) have already been described by García-Cobos et al. [10], while the remaining two were determined in this study and presented the Ala454→ Val and Asp350→ Asn/Thr532→ Asn substitutions.
Fourteen of 36 gBLNAR isolates (38.9%) presented ampicillin MIC within the susceptibility range (≤0.25–1 mg/L). All the isolates with MIC ≤0.25 or 0.5 of ampicillin belonged to the miscellaneous group, suggesting that these mutations were not involved in decreased β-lactam susceptibility.
Phenotypic and Genotypic Characterization
Phenotypically, the most common biotype found was biotype II (39.0%) followed by biotypes III (35.7%), I (16.8%), V (3.2%), VI (3.2%) and IV (2.1%). As a result of positive detection of lgtC, fucK and iga genes, all the isolates were identified as H. influenzae.
Molecular typing by PFGE revealed 47 different PFGE patterns. Twenty-six patterns were genotypically unique and 21 clusters contained between 2 and 15 related isolates (>80% similarity). Furthermore, molecular typing by MLST showed 67 different sequence types, with 28 of them (ST974, ST989 to ST1000, ST1143, ST1162, ST1163, ST1171, ST1172, ST1174, and ST1176 to ST1184) being described for the first time in the present study. The most frequent ST was ST159 (7 isolates). Analysis with e-BURST (including single and double locus variants) revealed 11 groups (≥2 isolates) and 29 singletons (only 1 isolate). Groups 1, 2 and 10 were the largest, with 9 isolates each (Table S1, Supplementary data).
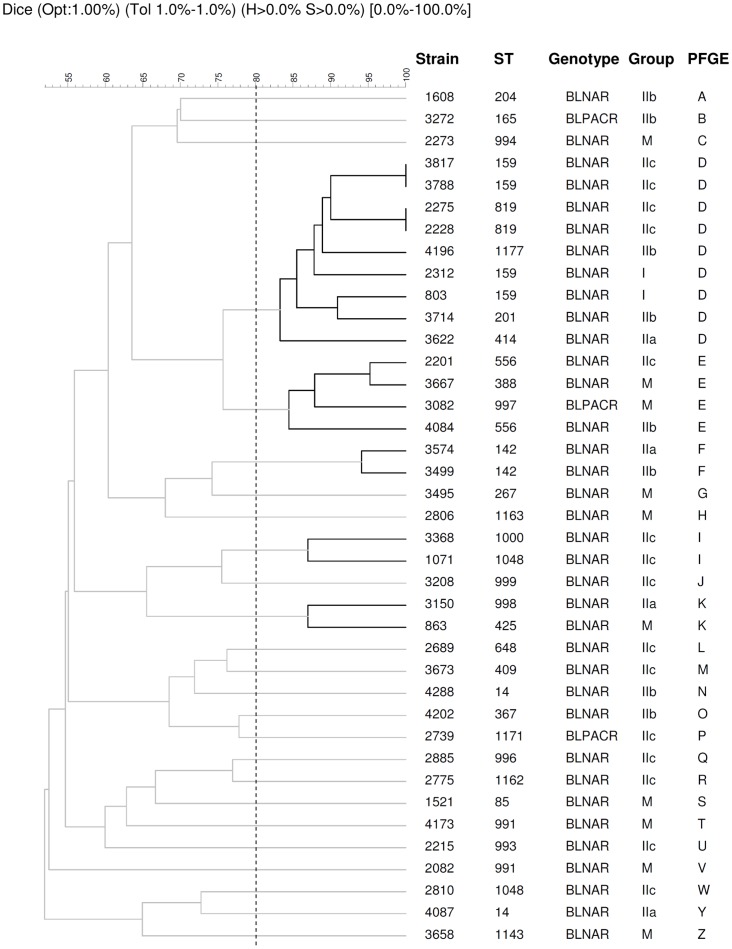
The 39 isolates with mutations in the ftsI gene were grouped into 25 independent PFGE clusters. Despite the fact that most patterns were unique, five clusters were identified with between two and nine genetically-related isolates (>80% similarity) (Figure 1). Cluster D grouped the majority of isolates with alterations in PBP3 (n = 9), with five different ST: ST159 (n = 4), ST819 (n = 2), ST201 (n = 1), ST414 (n = 1) and ST1177 (n = 1). These nine clonally-related isolates were collected from different patients throughout the study period. Six of these isolates were grouped in the same e-BURST group (ST159/ST819). The isolates in this cluster belonged to different amino acid substitution groups: IIc (n = 4), IIb (n = 2), I (n = 2) and IIa (n = I). Cluster E contained four isolates with three different ST: ST556 (n = 2), ST388 (n = 1) and ST997 (n = 1). Two of these isolates belonged to the miscellaneous group of amino acid substitutions, while the remaining two isolates belonged to subgroups IIb and IIc, respectively. The other three clusters (F, I and K) contained two isolates each. The isolates in cluster F had the same ST (ST142) and were classified into subgroups IIa and IIb. Cluster I comprised isolates with ST1000 and ST1048, which belonged to the same subgroup (IIc). Finally, cluster K was composed of isolates with ST425 and ST998, which were grouped into subgroup IIa and the miscellaneous amino acid substitution groups, respectively.
Figure 1. Tree diagram showing the genetic relatedness of 39 nontypeable H. influenzae isolates with mutations in the ftsI gene (gBLNAR n = 36 and gBLPACR n = 3) obtained by PFGE according to Dice's similarity index.
Dice coefficients are shown above the tree diagram. Isolates with ≥80% relatedness are considered highly genetically related.
Treatment and Patient Outcomes
Antibiotic therapy and clinical outcomes were analyzed for all patients included in this study. All patients were treated following the recommendations of the Infectious Disease Society of America and the guidelines of the American Thoracic Society [29].
Forty-one of 46 patients infected by gBLNAR, gBLPAR or gBLPACR isolates were successfully treated, mainly with amoxicillin/clavulanic acid, ceftriaxone and levofloxacin, or by using a combination of two of these antibiotics. The remaining five patients, infected by gBLNAR isolates, were treated with amoxicillin/clavulanic acid and ceftriaxone but died, due to aspiration, during the first 72 h of hospital admission (Table 3).
Table 3. Treatment and clinical outcomes for episodes of community-acquired pneumonia caused by NTHi.
| Genotypec | Outcome | Treatmenta | ||||
| AMC | CRO | LEV | SXT | Combined therapyb | ||
| gBLNAS | ||||||
| Cured | 46 | 12 | 19 | 5 | 1 | 9 |
| Died | 3 | 2 | 1 | |||
| gBLNAR | ||||||
| Cured | 31 | 11 | 15 | 4 | 6 | |
| Died | 5 | 3 | 2 | |||
| gBLPAR | ||||||
| Cured | 7 | 3 | 1 | 3 | ||
| gBLPACR | ||||||
| Cured | 3 | 1 | 1 | 1 | ||
a AMC: amoxicillin/clavulanic acid; CRO: ceftriaxone; LEV: levofloxacin; SXT: cotrimoxazole.
β-lactam with fluoroquinolone or fluoroquinolone with another antibiotic.b Combined therapy is
c Genotypes are defined in the Materials and Methods section.
Forty-three of 46 patients infected by isolates with a genotype susceptible to aminopenicillins (gBLNAS) were successfully treated with ceftriaxone, amoxicillin/clavulanic acid and levofloxacin. The remaining three patients died by aspiration or due to complication of their severe underlying diseases (Table 3).
Discussion
H. influenzae is a common cause of CAP in adults (6–10%) [8] and it is frequently associated with recurrent pneumonia in both children and adults [8], [30]. In this study, we analyzed the molecular epidemiology of NTHi causing non-bacteremic CAP in adult patients in the Barcelona area of Spain.
β-lactam antimicrobials are the first therapeutic option for treating CAP due to H. influenzae [29]. Resistance to ampicillin varies among European countries [31], [32]. The rate of reduced susceptibility to ampicillin found in this study was 33.7% (10.5% of isolates were resistant and 23.2% presented intermediate resistance), which is higher than the rate reported (16.2%) in a recent Spanish study by Perez-Trallero et al. [16]. A possible explanation for this high percentage of ampicillin non-susceptibility is that the majority of NTHi isolates were obtained from elderly patients who had received multiple antibiotic courses for their underlying diseases.
β-lactamase are the most common mechanism through which resistance to β-lactam antibiotics is acquired, although the frequency of their involvement fluctuates depending on the geographical area in question [33]–[35]. In our study, 10.5% of isolates presented TEM-1 β-lactamase production. This result is consistent with an overall downward trend that has been observed in Spain (from 25.7% in 1997 to 15.7% in 2007 [16]), as well as in other European countries and the USA [36]. However, different rates of β-lactam resistance due to alterations in PBP3 have been reported in several countries [15], [24], [36], [37]. In the present study, 41.1% of isolates had amino acid substitutions in the transpeptidase domain of PBP3. The percentage of BLNAR isolates detected in other European countries such as Germany (11.8%), France (0%), Portugal (9.6%) and the UK (1.5%) is lower than that found here [37]. The observed rate of gBLNAR could be due to the fact that most of our patients with CAP received multiple β-lactam antibiotic courses as treatment for their underlying diseases. Furthermore, the consumption of aminopenicillins in Catalonia increased from 46.1% in 1992 to 59.6% in 2007 [38], and this could also explain the frequency of gBLNAR observed in this study. In line with a previous report on Spanish isolates [10], the most frequent mutation found in the ftsI gene was Asn526→ Lys, followed by Arg517→ His, and this allowed us to use the Dabernat et al. classification to group our isolates [24]. The presence of these mutations conferred a reduced susceptibility on ampicillin and amoxicillin/clavulanic acid (MIC between 1–4 mg/L) although those mutations alone were not enough to confer full resistance. In this set of NTHi, no isolates were found to belong to groups III or III-like (Met377→ Ile and Ser385→ Thr substitutions), which have been related to decreased cefotaxime and cefixime susceptibility [10].
Most of our patients infected with strains that were non-susceptible to ampicillin were successfully treated with amoxicillin/clavulanic acid, ceftriaxone or levofloxacin. In accordance with other studies [16], [31], amoxicillin/clavulanic acid, third-generation cephalosporins and quinolones showed excellent in vitro activity and are good therapeutic options for treating non-bacteremic CAP due to NTHi. However, since no gBLNAR isolates with ampicillin MIC ≥4 mg/L were found in our study, the clinical outcomes of patients infected by strains with high ampicillin MIC is unknown.
NTHi strains isolated from CAP episodes were found to be genetically diverse, this being consistent with other surveillance studies performed on respiratory or invasive NTHi isolates [39], [40]. Some studies carried out on BLNAR strains have demonstrated the high genotypic heterogeneity and lack of clonal spread in these strains [41], [42]. However, recent studies suggest a clonal dissemination of some BLNAR or BLPACR strains [10], [43], [44]. In our study, some small clusters of gBLNAR strains were found (Figure 1), but only one cluster, comprising two strains, presented the same ftsI pattern, thereby suggesting a lack of clonal distribution in NTHi from CAP patients.
In conclusion, this study has established the genotypic characterization and antimicrobial resistance of NTHi causing non-bacteremic CAP in adult patients. The results illustrate the high genetic diversity among these strains, as well as the high rate of reduced susceptibility to ampicillin due to alterations in PBP3. Finally, the analysis of treatment and outcomes in this group of patients demonstrated that NTHi strains with mutations in the ftsI gene (gBLNAR and gBLPACR) could be successfully treated with ceftriaxone or fluoroquinolones.
Supporting Information
Groups based on e-BURST analysis with MLST data of 95 NTHi causing non-bacteremic CAP.
(DOC)
Acknowledgments
We acknowledge use of the Haemophilus influenzae MLST website. We wish to thank all the staff of the Microbiology Laboratory of Hospital Universitari de Bellvitge who contributed to this project on a daily basis.
Funding Statement
This study was supported by a grant from the Fondo de Investigaciones Sanitarias de la Seguridad Social (PI0901904), by CIBER de Enfermedades Respiratorias (CIBERES - CB06/06/0037), run by the ISCIII (Instituto de Salud Carlos III), Madrid, Spain, and by Spanish Network for Research on Infectious Diseases (REIPI, RD12/0015), run by ISCIII, Madrid, Spain. CP was supported by grants from AGAUR-FI (Generalitat de Catalunya, Spain) and from FPU (Formación de Profesorado Universitario, Ministerio de Educación, Spain). SM was supported by a “Sara Borrell Postdoctoral Contract, CD10/00298” from the Instituto de Salud Carlos III (ISCIII), Madrid, Spain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Agrawal A, Murphy TF (2011) Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 49: 3728–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eldika N, Sethi S (2006) Role of nontypeable Haemophilus influenzae in exacerbations and progression of chronic obstructive pulmonary disease. Curr Opin Pulm Med 12: 118–124. [DOI] [PubMed] [Google Scholar]
- 3. Erwin AL, Smith AL (2007) Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol 15: 355–362. [DOI] [PubMed] [Google Scholar]
- 4. Murphy TF, Faden H, Bakaletz LO, Kyd JM, Forsgren A, et al. (2009) Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J 28: 43–48. [DOI] [PubMed] [Google Scholar]
- 5. Saito A, Kohno S, Matsushima T, Watanabe A, Oizumi K, et al. (2006) Prospective multicenter study of the causative organisms of community-acquired pneumonia in adults in Japan. J Infect Chemother 12: 63–69. [DOI] [PubMed] [Google Scholar]
- 6. Viasus D, Garcia-Vidal C, Castellote J, Adamuz J, Verdaguer R, et al. (2011) Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine (Baltimore) 90: 110–118. [DOI] [PubMed] [Google Scholar]
- 7. Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J (2010) Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 50: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Vidal C, Carratala J, Fernandez-Sabe N, Dorca J, Verdaguer R, et al. (2009) Aetiology of, and risk factors for, recurrent community-acquired pneumonia. Clin Microbiol Infect 15: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 9. Bell SM, Plowman D (1980) Mechanisms of ampicillin resistance in Haemophilus influenzae from respiratory tract. Lancet 1: 279–280. [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Cobos S, Campos J, Lazaro E, Roman F, Cercenado E, et al. (2007) Ampicillin-resistant non-beta-lactamase-producing Haemophilus influenzae in Spain: recent emergence of clonal isolates with increased resistance to cefotaxime and cefixime. Antimicrob Agents Chemother 51: 2564–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tristram S, Jacobs MR, Appelbaum PC (2007) Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev 20: 368–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scriver SR, Walmsley SL, Kau CL, Hoban DJ, Brunton J, et al. (1994) Determination of antimicrobial susceptibilities of Canadian isolates of Haemophilus influenzae and characterization of their beta-lactamases. Canadian Haemophilus Study Group. Antimicrob Agents Chemother 38: 1678–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendelman PM, Chaffin DO, Stull TL, Rubens CE, Mack KD, et al. (1984) Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother 26: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parr TR Jr, Bryan LE (1984) Mechanism of resistance of an ampicillin-resistant, beta-lactamase-negative clinical isolate of Haemophilus influenzae type b to beta-lactam antibiotics. Antimicrob Agents Chemother 25: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ubukata K, Shibasaki Y, Yamamoto K, Chiba N, Hasegawa K, et al. (2001) Association of amino acid substitutions in penicillin-binding protein 3 with beta-lactam resistance in beta-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother 45: 1693–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez-Trallero E, Martin-Herrero JE, Mazon A, Garcia-Delafuente C, Robles P, et al. (2010) Antimicrobial resistance among respiratory pathogens in Spain: latest data and changes over 11 years (1996–1997 to 2006–2007). Antimicrob Agents Chemother 54: 2953–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roson B, Carratala J, Verdaguer R, Dorca J, Manresa F, et al. (2000) Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis 31: 869–874. [DOI] [PubMed] [Google Scholar]
- 18. Binks MJ, Temple B, Kirkham LA, Wiertsema SP, Dunne EM, et al. (2012) Molecular surveillance of true nontypeable Haemophilus influenzae: an evaluation of PCR screening assays. PLoS One 7: e34083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kilian M (1976) A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol 93: 9–62. [DOI] [PubMed] [Google Scholar]
- 20. Falla TJ, Crook DW, Brophy LN, Maskell D, Kroll JS, et al. (1994) PCR for capsular typing of Haemophilus influenzae. J Clin Microbiol 32: 2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wayne P (2006) Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Growth Aerobically. Approved Standard M7–A6.
- 22.Wayne P (2010) Clinical Laboratory Standard Institute (CLSI). Performance standards for antimicrobial susceptibility testing: Twentieth informational supplement. CLSI document M100–S20.
- 23. Tenover FC, Huang MB, Rasheed JK, Persing DH (1994) Development of PCR assays to detect ampicillin resistance genes in cerebrospinal fluid samples containing Haemophilus influenzae. J Clin Microbiol 32: 2729–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dabernat H, Delmas C, Seguy M, Pelissier R, Faucon G, et al. (2002) Diversity of beta-lactam resistance-conferring amino acid substitutions in penicillin-binding protein 3 of Haemophilus influenzae. Antimicrob Agents Chemother 46: 2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia-Cobos S, Campos J, Cercenado E, Roman F, Lazaro E, et al. (2008) Antibiotic resistance in Haemophilus influenzae decreased, except for beta-lactamase-negative amoxicillin-resistant isolates, in parallel with community antibiotic consumption in Spain from 1997 to 2007. Antimicrob Agents Chemother 52: 2760–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim IS, Ki CS, Kim S, Oh WS, Peck KR, et al. (2007) Diversity of ampicillin resistance genes and antimicrobial susceptibility patterns in Haemophilus influenzae strains isolated in Korea. Antimicrob Agents Chemother 51: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hotomi M, Kono M, Togawa A, Arai J, Takei S, et al. (2010) Haemophilus influenzae and Haemophilus haemolyticus in tonsillar cultures of adults with acute pharyngotonsillitis. Auris Nasus Larynx 37: 594–600. [DOI] [PubMed] [Google Scholar]
- 28. Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, et al. (2003) Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol 41: 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, et al. (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44 Suppl 2S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Schutter I, De Wachter E, Crokaert F, Verhaegen J, Soetens O, et al. (2011) Microbiology of bronchoalveolar lavage fluid in children with acute nonresponding or recurrent community-acquired pneumonia: identification of nontypeable Haemophilus influenzae as a major pathogen. Clin Infect Dis 52: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blosser-Middleton R, Sahm DF, Thornsberry C, Jones ME, Hogan PA, et al. (2003) Antimicrobial susceptibility of 840 clinical isolates of Haemophilus influenzae collected in four European countries in 2000–2001. Clin Microbiol Infect 9: 431–436. [DOI] [PubMed] [Google Scholar]
- 32. Morrissey I, Maher K, Williams L, Shackcloth J, Felmingham D, et al. (2008) Non-susceptibility trends among Haemophilus influenzae and Moraxella catarrhalis from community-acquired respiratory tract infections in the UK and Ireland, 1999–2007. J Antimicrob Chemother 62 Suppl 2ii97–103. [DOI] [PubMed] [Google Scholar]
- 33. Critchley IA, Brown SD, Traczewski MM, Tillotson GS, Janjic N (2007) National and regional assessment of antimicrobial resistance among community-acquired respiratory tract pathogens identified in a 2005–2006 U.S. Faropenem surveillance study. Antimicrob Agents Chemother 51: 4382–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fluit AC, Florijn A, Verhoef J, Milatovic D (2005) Susceptibility of European beta-lactamase-positive and -negative Haemophilus influenzae isolates from the periods 1997/1998 and 2002/2003. J Antimicrob Chemother 56: 133–138. [DOI] [PubMed] [Google Scholar]
- 35. Wang H, Chen M, Xu Y, Sun H, Yang Q, et al. (2011) Antimicrobial susceptibility of bacterial pathogens associated with community-acquired respiratory tract infections in Asia: report from the Community-Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study, 2009–2010. Int J Antimicrob Agents 38: 376–383. [DOI] [PubMed] [Google Scholar]
- 36. Heilmann KP, Rice CL, Miller AL, Miller NJ, Beekmann SE, et al. (2005) Decreasing prevalence of beta-lactamase production among respiratory tract isolates of Haemophilus influenzae in the United States. Antimicrob Agents Chemother 49: 2561–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jansen WT, Verel A, Beitsma M, Verhoef J, Milatovic D (2006) Longitudinal European surveillance study of antibiotic resistance of Haemophilus influenzae. J Antimicrob Chemother 58: 873–877. [DOI] [PubMed] [Google Scholar]
- 38. Llor C, Cots JM, Gaspar MJ, Alay M, Rams N (2009) Antibiotic prescribing over the last 16 years: fewer antibiotics but the spectrum is broadening. Eur J Clin Microbiol Infect Dis 28: 893–897. [DOI] [PubMed] [Google Scholar]
- 39. Saito M, Umeda A, Yoshida S (1999) Subtyping of Haemophilus influenzae strains by pulsed-field gel electrophoresis. J Clin Microbiol 37: 2142–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shuel M, Law D, Skinner S, Wylie J, Karlowsky J, et al. (2010) Characterization of nontypeable Haemophilus influenzae collected from respiratory infections and invasive disease cases in Manitoba, Canada. FEMS Immunol Med Microbiol 58: 277–284. [DOI] [PubMed] [Google Scholar]
- 41. Gazagne L, Delmas C, Bingen E, Dabernat H (1998) Molecular epidemiology of ampicillin-resistant non-beta-lactamase-producing Haemophilus influenzae. J Clin Microbiol 36: 3629–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mendelman PM, Chaffin DO, Musser JM, De GR, Serfass DA, et al. (1987) Genetic and phenotypic diversity among ampicillin-resistant, non-beta-lactamase-producing, nontypeable Haemophilus influenzae isolates. Infect Immun 55: 2585–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barbosa AR, Giufre M, Cerquetti M, Bajanca-Lavado MP (2011) Polymorphism in ftsI gene and {beta}-lactam susceptibility in Portuguese Haemophilus influenzae strains: clonal dissemination of beta-lactamase-positive isolates with decreased susceptibility to amoxicillin/clavulanic acid. J Antimicrob Chemother 66: 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Resman F, Ristovski M, Forsgren A, Kaijser B, Kronvall G, et al. (2012) Increase of beta-Lactam-Resistant Invasive Haemophilus influenzae in Sweden, 1997 to 2010. Antimicrob Agents Chemother 56: 4408–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Groups based on e-BURST analysis with MLST data of 95 NTHi causing non-bacteremic CAP.
(DOC)