Abstract
Objective
End-stage renal disease (ESRD) is a severe health concern over the world. Associations between apolipoprotein E (apoE) gene polymorphisms and the risk of ESRD remained inconclusive. This study aimed to investigate the association between apoE gene polymorphisms and ESRD susceptibility.
Methods
Databases including PubMed, Embase, Web of Science and the Cochrane Library were searched to find relevant studies. Meta-analysis method was used synthesize the eligible studies.
Results
Sixteen pertinent case-control studies which included 3510 cases and 13924 controls were analyzed. A significant association was found between ε2 allele and the ESRD risk (odds ratio (OR) = 1.30, 95% confidence interval (CI) 1.15–1.46, P < 0.0001; I 2 = 18%, P for heterogeneity = 0.24). The ε2ε3, ε2ε4, ε3ε3, ε3ε4, ε4ε4, ε3 and ε4 were not associated with the susceptibility of ESRD. In the subgroup analysis by ethnicity, there was a statistically significant association between ε2ε3 or ε2 allele and ESRD risk in East Asians (OR = 1.66, 95% CI 1.31–2.10, P < 0.0001; OR = 1.62, 95% CI 1.31–2.01, P < 0.0001, respectively), but not in Caucasians. E2 carriers had higher plasma apoE (mean difference = 16.24 mg/L, 95% CI 7.76-24.73, P = 0.0002) than the (ε3 + ε4) carriers in patients with ESRD. The publication bias was not significant.
Conclusion
The ε2 allele of apoE gene might increase the risk of ESRD. E2 carriers expressed higher level of plasma apoE in patients with ESRD. More well-designed studies are needed to confirm these associations in the future.
Introduction
End-stage renal disease (ESRD) is a significant public health problem in the world. The etiology of ESRD is not clear yet. In addition to dyslipidemia, chronic glomerulonephritis, hypertension and diabetes, ESRD is a multifaceted disorder with inherited components playing an important role [1].
Apolipoprotein E (apoE) is a component of lipoprotein, and is one of the key regulatory proteins in cholesterol and lipoprotein metabolism. It is also the structural protein of chylomicron (CM) remnants, low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and high-density lipoprotein (HDL) [2]. ApoE acts as the ligand of the LDL receptor and apoE receptor, and is synthesized in kidney, liver and adrenal cortex [3]. The apoE knockout mice represented hyperlipidemia, accelerated atherogenesis, and glomerulosclerosis-like lesion [3]. ApoE gene polymorphisms and proteins played important roles in the pathogenesis of ESRD [4]. First, patients with high level of apoE2 were more likely to develop into chronic kidney disease (CKD) or even ESRD, because the clearances of VLDL and CM remnants were delayed by apoE2 [5]. Second, ApoE had a high affinity with extracellular glycosaminoglycans which were binded to many growth factors (e.g. tumor growth factor β and platelet-derived growth factor) [6]. ApoE may increase the risk of kidney injury or ESRD by up-regulating the growth factors. Third, lipoprotein glomerulopathy (LPG) was found to be associated with apoEs and the ε2 phenotype [7]. Patients with LPG had a high level of apoE in the serum and capillary thrombosis.
ApoE gene locates at 19q13.2, which includes 4 exons and 3 introns. Gene polymorphism was determined by the different amino acid residues at 112 and 158 sites in exon 4. ApoE gene polymorphisms include three codominant alleles: ε2, ε3, ε4, which encode 3 protein isoforms (E2, E3 and E4) [8]. ApoE2 had the worst binding affinity with the apoE receptor among them [9]. Among the 3 alleles, either 2 of the alleles can produce 6 different phenotypes in total: 3 homozygotes (ε2ε2, ε3ε3, ε4ε4) and 3 heterozygotes (ε2ε3, ε2ε4, ε3ε4). Allele frequency from high to low is: ε3, ε2, ε4, and ε3ε3 is the most common phenotype in human [10]. It is commonly believed the ε2 allele was associated with the progressive decline of renal function, and ε4 reduced the risk of ESRD [11,12]. In Hubacek et al.’s study [13], patients in hemodialysis had higher frequency of ε2 than the control group (15.9% vs. 12.2%). However, Roussos et al. [14] found patients with ESRD had no difference of ε2, ε3 and ε4 distribution compared with the control group. Feussner et al. also failed to find the association between ε2 and ESRD [15].
The results of the studies remained inconsistent. This meta-analysis was performed to investigate the precise role of apoE gene variants on the risk of ESRD.
Methods
Information sources and Search
Pubmed, EMBASE, Web of Science and the Cochrane library were all searched (published up to May, 2013). The terms in electronic search included “apoE”, “apolipoprotein E”, “ESRD or end-stage renal disease”, “chronic renal failure”, “dialysis”, “polymorphism or mutation or variant”. In addition, Google Scholar was used to check the references of eligible trials to make sure all studies were included.
Inclusion and Exclusion Criteria
Studies fulfilling the following selection criteria were included in this meta-analysis: (1) the outcome had to be ESRD; (2) using case-control design, and control group were unrelated people chosen randomly from the same geographic region; (3) genotype distributions should be available for estimating an odds ratio (OR) and 95% confidence interval (CI) in both cases and controls. If one of the following existed, studies were excluded: (1) not relevant to apoE polymorphisms or ESRD; (2) study design based on sibling or family pairs; (3) neither genotype frequencies nor number reported; (4) reviews or case reports.
Study Selection and data extraction
Included studies were independently reviewed by two investigators. Relevant data was extracted into predesigned data collection forms. If there was any discrepancy, two investigators resolved it by discussion or a third author would assess the relevant studies. We collected the following data from each included trial: first author’s name, year of publication, country, ethnicity, sample size, age, genotyping method, ESRD treatment, and genotype number in cases and controls.
Statistical Analysis
The strength of the association between apoE polymorphisms and ESRD risk was measured by OR and 95% CI. When P value < 0.05, OR was considered statistically significant. Continuous outcome data from included trials were analyzed using the mean difference (WMD) and 95% CI. The heterogeneity of identified studies was assessed by I 2 and the Chi square-test based Cochrane Q-test. When P value > 0.10 for the Q test, it indicated a lack of heterogeneity among the studies. Then the fixed-effects model was used to pool OR. Otherwise, OR was pooled in the random-effects model. The sources of the heterogeneity and ethnic-specific effect were evaluated by subgroup analyses performed by ethnicity. One-way sensitivity analysis was carried out to access the stability of the meta-analysis. We also excluded the studies not in Hardy-Weinberg equilibrium (HWE) to perform the sensitivity analysis. Cumulative meta-analysis was made by sequential random-effects pooling (starting with the earliest studies), in order to show the consequence of adding studies on the effect size. HWE in controls was tested by the Chi-square test [16]. The Begg’s test and Egger’s test were used to assess publication bias [17]. When performing a series of comparisons in the same sample, the bonferroni correction of critical P values was used. Revman 5.1 software (Nordic Cochrane Center, Copenhagen, Denmark) and STATA 11.0 software (Stata Corporation, College Station, TX) were used to perform all the statistical tests.
Results
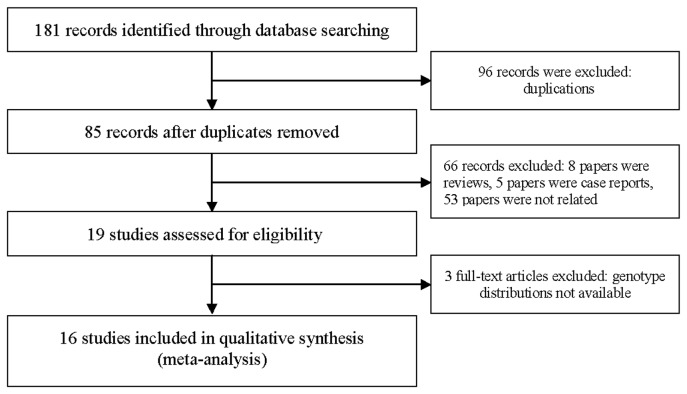
A total of 16 clinical studies [14,15,18-31] on ESRD and apoE variants published from 1992 and 2008 were identified, among which seven studies were form Asia [19,22-24,27,29,31], eight from Europe [14,15,20,21,25,26,28,30], and one from North America [18]. The literature review process was shown in Figure 1 . Among the 16 studies, six studies were performed in East Asians [19,22-24,27,29], and 10 in Caucasians [14,15,18,20,21,25,26,28,30,31] (Table 1 ). Table S1 showed the PRISMA 2009 Checklist. Figure S1 showed the PRISMA 2009 Flow Diagram. Renal replacement therapies in patients with ESRD included continuous ambulatory peritoneal dialysis (CAPD), hemodialysis (HD) or HD plus CAPD. Two included studies used CAPD in the ESRD group [18,22], four using HD plus CAPD [14,24,29,30], and 10 using HD [15,19-21,23,25-28,31]. All the studies were performed in adults. HWE examination results and genotype frequencies were listed in Table 2 . All the studies fit the HWE except three articles [18,23,25].
Figure 1. Flow chart of the study identification, inclusion, and exclusion.
Table 1. Characteristics of included trials.
| Author /Year | Country | Ethnicity | Age group | Case (n) | Control (n) | Male/Female of ESRD (n) | Dialysis type | Genotyping method |
|---|---|---|---|---|---|---|---|---|
| Feussner/1992 | Germany | Caucasian | Adults | 560 | 1031 | NA | HD | PCR |
| Eggertsen/1997 | USA | Caucasian | Adults | 51 | 407 | 25/26 | CAPD | PCR |
| Olmer/1997 | France | Caucasian | Adults | 66 | 338 | 33/33 | HD | PCR |
| Fumitake/1997 | Japan | East Asians | Adults | 97 | 173 | 58/39 | HD | PCR |
| Kohlmeier/1998 | Germany | Caucasian | Adults | 219 | 1031 | NA | HD | PCR |
| Choi/1999 | Korea | East Asians | Adults | 54 | 194 | 27/27 | CAPD | PCR |
| Oda/1999 | Japan | East Asians | Adults | 485 | 576 | NA | CAPD+HD | PCR- RFLP |
| Imura/1999 | Japan | East Asians | Adults | 493 | 422 | 287/206 | HD | PCR |
| Guz/2000 | Turkey | Caucasian | Adults | 269 | 8366 | 154/115 | HD | PCR |
| Jana/2002 | Czech republic | Caucasian | Adults | 87 | 67 | 53/34 | HD | PCR- RFLP |
| Asakimori/2003 | Japan | East Asians | Adults | 163 | 576 | 85/78 | HD | PCR |
| Roussos/2004 | Sweden | Caucasian | Adults | 385 | 407 | 220/165 | CAPD+HD | PCR |
| Liberopoulos/2004 | Greece | Caucasian | Adults | 301 | 200 | 168/133 | HD | PCR |
| Huang/2005 | China | East Asians | Adults | 94 | 108 | 54/40 | CAPD+HD | PCR |
| Arikan/2007 | Turkey | Caucasian | Adults | 144 | 42 | 49/70 | CAPD+HD | PCR |
| Fahad/2008 | Saudi Arabia | Caucasian | Adults | 42 | 50 | NA | HD | PCR |
CAPD: continuous ambulatory peritoneal dialysis; HD: hemodialysis; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; NA, not available.
Table 2. Distribution of apoE polymorphism among patients with ESRD and controls.
| Studies | ESRD |
Control |
Hardy–Weinberg equilibrium | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε2ε2 | ε2ε3 | ε2ε4 | ε3ε3 | ε3ε4 | ε4ε4 | ε2ε2 | ε2ε3 | ε2ε4 | ε3ε3 | ε3ε4 | ε4ε4 | |||
| Feussner/1992 | 8 | 60 | 18 | 346 | 117 | 11 | 10 | 124 | 15 | 617 | 236 | 29 | Yes | |
| Olmer/1997 | 1 | 8 | 1 | 41 | 15 | 0 | 3 | 40 | 4 | 231 | 54 | 6 | Yes | |
| Fumitake/1997 | 0 | 10 | 2 | 66 | 19 | 1 | 0 | 15 | 0 | 131 | 24 | 3 | Yes | |
| Eggertsen/1997 | 0 | 2 | 3 | 30 | 14 | 2 | 4 | 42 | 14 | 239 | 65 | 43 | No | |
| Kohlmeier/1998 | 7 | 31 | 1 | 139 | 39 | 2 | 10 | 124 | 15 | 617 | 236 | 29 | Yes | |
| Oda/1999 | 1 | 50 | 4 | 347 | 82 | 1 | 2 | 35 | 4 | 414 | 111 | 10 | Yes | |
| Choi/1999 | 0 | 8 | 0 | 37 | 9 | 0 | 0 | 16 | 0 | 147 | 31 | 0 | Yes | |
| Imura/1999 | 0 | 53 | 0 | 350 | 90 | 0 | 0 | 41 | 0 | 316 | 65 | 0 | No | |
| Guz/2000 | 4 | 33 | 2 | 200 | 28 | 2 | 33 | 887 | 67 | 6208 | 1079 | 92 | No | |
| Jana/2002 | 1 | 9 | 3 | 63 | 11 | 0 | 0 | 9 | 1 | 44 | 12 | 1 | Yes | |
| Asakimori/2003 | 1 | 21 | 1 | 109 | 31 | 0 | 2 | 35 | 4 | 414 | 111 | 10 | Yes | |
| Roussos/2004 | 1 | 49 | 9 | 215 | 96 | 15 | 1 | 42 | 9 | 181 | 101 | 9 | Yes | |
| Liberopoulos /2004 | 5 | 28 | 3 | 224 | 40 | 1 | 0 | 21 | 2 | 128 | 44 | 5 | Yes | |
| Huang/2005 | 4 | 30 | 7 | 47 | 6 | 0 | 2 | 15 | 4 | 76 | 11 | 0 | Yes | |
| Arikan/2007 | 1 | 18 | 4 | 107 | 12 | 2 | 1 | 3 | 0 | 36 | 2 | 0 | Yes | |
| Fahad/2008 | 0 | 4 | 1 | 33 | 3 | 1 | 0 | 3 | 0 | 35 | 11 | 1 | Yes | |
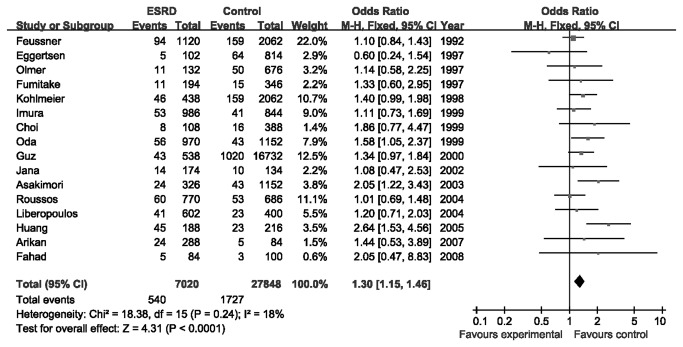
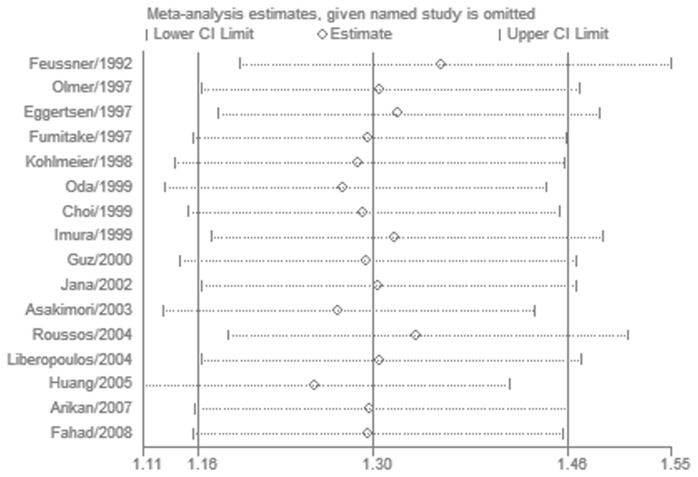
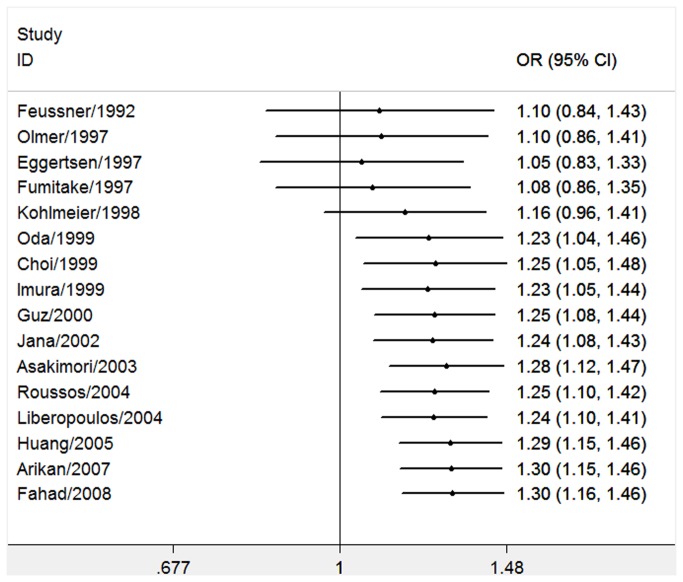
The eligible studies [14,15,18-31] included 3510 ESRD cases and 13924 controls. According to the bonferroni correction of critical P values, the results about apoE gene polymorphism were considered to be statistically significant when P < 0.00018. A significant association was found between ε2 allele and the ESRD risk (OR = 1.30, 95% CI 1.15–1.46, P < 0.0001; I 2 = 18%, P for heterogeneity = 0.24) (Figure 2 ). The ε2ε3, ε2ε4, ε3ε3, ε3ε4, ε4ε4, ε3 and ε4 were not associated with the susceptibility of ESRD (Table 3 ). Interestingly, both ε4ε4 and ε4 allele showed lower risk of ESRD than the control group (OR = 0.55, 95% CI 0.38–0.81, P = 0.002; OR = 0.86, 95% CI 0.75–0.99, P = 0.04, respectively), but the P values did not reach the statistical criterion. Then in the results of subgroup analysis by ethnicity, there was a statistically significant association between ε2ε3 or ε2 allele and ESRD risk in East Asians [19,22-24,27,29] (OR = 1.66, 95% CI 1.31–2.10, P < 0.0001, P for heterogeneity = 0.18; OR = 1.62, 95% CI 1.31–2.01, P < 0.0001, P for heterogeneity = 0.20, respectively) (Table 3 ). However, we did not find significant association between ε2 and ESRD in Caucasians (OR = 1.17, 95% CI 1.02–1.36, P = 0.03; I 2 = 0%, P for heterogeneity = 0.81) [14,15,18,20,21,25,26,28,30,31]. In consideration of our conserved P value, the positive association still could not be excluded in Caucasians. More investigations about Caucasians should be performed in the future. The heterogeneity decreased a lot in the subgroup analysis 1-way sensitivity analysis was performed to evaluate the stability of the meta-analysis of ε2 allele (Figure 3 ). When any single study was omitted, the significance of the results did not change. We also conducted the cumulative meta-analyses of ε2 allele. Figure 4 showed the inclination of ε2 allele toward significant association with ESRD risk. Then we excluded the studies [18,23,25] not in HWE in the sensitivity analysis. All the results kept consistent with the primary ones (Table 4 ). The heterogeneity of the ε3ε4 was improved (I 2 = 23%, P = 0.21).
Figure 2. Meta-analysis for the association between the ε2 allele and ESRD risk.
Table 3. Determination of the genetic effects of apoE polymorphisms on ESRD and subgroup analyses.
| Studies number | Heterogeneity (P value) | Model | OR (95 % CI) | P value | |
|---|---|---|---|---|---|
| Genetic contrasts | |||||
| ε2ε2+ versus ε2ε2- | 16 | 0.78 | Fixed | 2.03 (1.27, 3.26) | 0.003 |
| ε2ε3+ versus ε2ε3- | 16 | 0.06 | Random | 1.25 (1.03, 1.51) | 0.02 |
| ε2ε4+ versus ε2ε4- | 16 | 0.88 | Fixed | 1.43 (0.99, 2.06) | 0.05 |
| ε3ε3+ versus ε3ε3- | 16 | 0.02 | Random | 0.96 (0.83, 1.10) | 0.56 |
| ε3ε4+ versus ε3ε4- | 16 | 0.05 | Random | 0.91 (0.78, 1.07) | 0.27 |
| ε4ε4+ versus ε4ε4- | 16 | 0.41 | Fixed | 0.55 (0.38, 0.81) | 0.002 |
| ε2+ versus ε2 - | 16 | 0.24 | Fixed | 1.30 (1.15, 1.46) | 0.0001 |
| ε3+ versus ε3- | 16 | 0.05 | Random | 0.97 (0.87, 1.08) | 0.57 |
| ε4+ versus ε4- | 16 | 0.06 | Random | 0.86 (0.75, 0.99) | 0.04 |
| East Asians | |||||
| ε2ε2+ versus ε2ε2- | 6 | 0.65 | Fixed | 1.52 (0.47, 4.90) | 0.48 |
| ε2ε3+ versus ε2ε3- | 6 | 0.18 | Fixed | 1.66 (1.31, 2.10) | 0.0001 |
| ε2ε4+ versus ε2ε4- | 6 | 0.76 | Fixed | 1.63 (0.73, 3.65) | 0.23 |
| ε3ε3+ versus ε3ε3- | 6 | 0.18 | Fixed | 0.81 (0.69, 0.94) | 0.007 |
| ε3ε4+ versus ε3ε4- | 6 | 0.45 | Fixed | 1.02 (0.84, 1.23) | 0.86 |
| ε4ε4+ versus ε4ε4- | 6 | 0.56 | Fixed | 0.19 (0.05, 0.73) | 0.01 |
| ε2+ versus ε2 - | 6 | 0.20 | Fixed | 1.62 (1.31, 2.01) | 0.0001 |
| ε3+ versus ε3- | 6 | 0.09 | Random | 0.81 (0.66, 1.00) | 0.05 |
| ε4+ versus ε4- | 6 | 0.26 | Fixed | 0.94 (0.79, 1.11) | 0.45 |
| Caucasians | |||||
| ε2ε2+ versus ε2ε2- | 10 | 0.64 | Fixed | 2.16 (1.29, 3.61) | 0.003 |
| ε2ε3+ versus ε2ε3- | 10 | 0.75 | Fixed | 1.01 (0.85, 1.20) | 0.90 |
| ε2ε4+ versus ε2ε4- | 10 | 0.72 | Fixed | 1.38 (0.92, 2.08) | 0.12 |
| ε3ε3+ versus ε3ε3- | 10 | 0.33 | Fixed | 1.11 (0.99, 1.24) | 0.09 |
| ε3ε4+ versus ε3ε4- | 10 | 0.04 | Random | 0.85 (0.68, 1.07) | 0.17 |
| ε4ε4+ versus ε4ε4- | 10 | 0.47 | Fixed | 0.64 (0.43, 0.96) | 0.03 |
| ε2+ versus ε2 - | 10 | 0.81 | Fixed | 1.17 (1.02, 1.36) | 0.03 |
| ε3+ versus ε3- | 10 | 0.43 | Fixed | 1.06 (0.96, 1.17) | 0.25 |
| ε4+ versus ε4- | 10 | 0.06 | Random | 0.81 (0.67, 0.98) | 0.03 |
Figure 3. One-way sensitivity analysis for the ε2 allele and ESRD risk.
Figure 4. Cumulative meta-analysis of associations between the ε2 allele and ESRD risk.
Table 4. Sensitivity analysis of the association between apoE polymorphisms and ESRD.
| Sensitivity analysis | Studies number | Heterogeneity (P value) | Model | OR (95 % CI) | P value |
|---|---|---|---|---|---|
| ε2ε2+ versus ε2ε2- | 13 | 0.79 | Fixed | 1.91 (1.13, 3.22) | 0.02 |
| ε2ε3+ versus ε2ε3- | 13 | 0.04 | Random | 1.32 (1.04, 1.66) | 0.02 |
| ε2ε4+ versus ε2ε4- | 13 | 0.81 | Fixed | 1.46 (0.98, 2.17) | 0.06 |
| ε3ε3+ versus ε3ε3- | 13 | 0.009 | Random | 0.96 (0.80, 1.14) | 0.64 |
| ε3ε4+ versus ε3ε4- | 13 | 0.21 | Fixed | 0.85 (0.74, 0.96) | 0.01 |
| ε4ε4+ versus ε4ε4- | 13 | 0.30 | Fixed | 0.57 (0.38, 0.86) | 0.007 |
| ε2+ versus ε2 - | 13 | 0.24 | Fixed | 1.34 (1.17, 1.54) | 0.0001 |
| ε3+ versus ε3- | 13 | 0.03 | Random | 0.96 (0.84, 1.11) | 0.61 |
| ε4+ versus ε4- | 13 | 0.08 | Random | 0.83 (0.71, 0.97) | 0.02 |
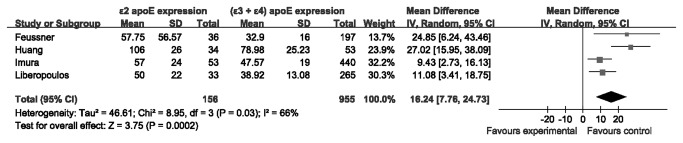
To investigate whether ε2 allele have higher expression of plasma apoE than the (ε3 + ε4) phenotypes in patients with ESRD, we performed another meta-analysis. Ε2 carriers with ESRD had increased apoE expression (4 studies [15,23,28,29], WMD = 16.24 mg/L, 95% CI 7.76-24.73, P = 0.0002; I 2 = 66%; P for heterogeneity = 0.03) than the (ε3 + ε4) carriers (Figure 5 ).
Figure 5. Meta-analysis for the plasma apoE between ε2 carriers and (ε3 + ε4) carriers in the patients with ESRD.
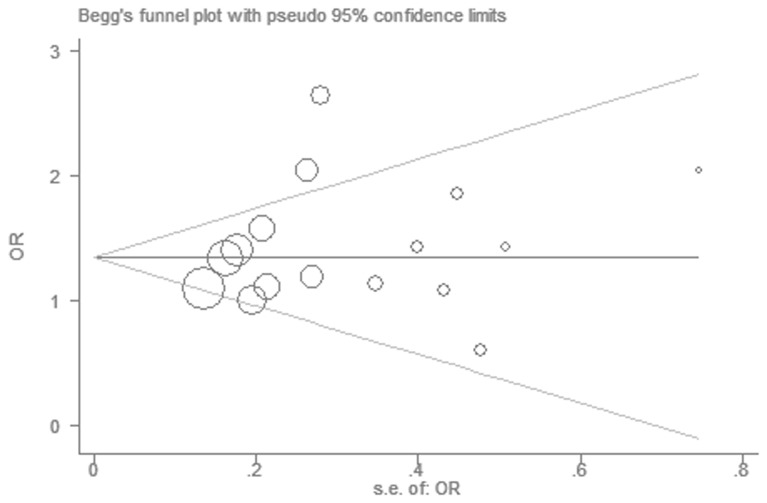
There was no significant publication bias in the Begg’s test (P = 0.344) and Egger’s test (P = 0.352). The funnel plot was symmetrical (Figure 6 ).
Figure 6. Funnel plot for the ε2 allele and ESRD risk.
Discussion
Several previous studies found the ε2 allele was a possible genetic risk factor for all-cause ESRD. Oda et al. [24] found apoE2 had higher frequency in patients with ESRD, and lower frequency of apoE4 was found. Patients carrying ε2 allele were associated with massive proteinuria [24]. Hubacek et al. found the strength of the OR (ε2) increased with the hemodialysis time of the patients. The ε2 allele may have different functions in different stages of ESRD [13]. Hsu et al. [32] proved apoE polymorphism could predict the progression of CKD independently, and increase the risk of early CKD manifestations such as high serum creatinine and macroalbuminuria. Recently, Chu et al. [33] found the ε2 allele was associated with lower levels of continuous GFR in non-Hispanic blacks. By the way, the ε2 allele in patients with type 2 diabetes is a risk factor for development of diabetic nephropathy. In the meta-analysis between apoE polymorphism and NS, Zhou et al. [10] found the apoE polymorphisms were associated with the susceptibility of NS. The ε2 allele may be a risk factor of renal disease.
In this meta-analysis, the association between the apoE polymorphisms and ESRD risk was explored. Sixteen eligible case-control studies which included 3510 cases and 13924 controls were analyzed. The results indicated that individuals with the ε2 allele showed an increased risk of ESRD in the overall population. Compared with those individuals with the ε3 and ε4 alleles, carriers of ε2 genotype had 30% increased risk of ESRD. Previous studies found the ε4 allele tended to be associated with lower odds of ESRD. However, we did not find the association after the meta-analysis. The results of different plasma apoE expression in the patients with ESRD indicated that ε2 carriers had 16.24 mg/L higher expression of plasma apoE than the (ε3 + ε4) carriers. In conclusion, the ε2 allele might increase the risk of ESRD and express more apoE protein. Early screening of the ε2 allele might prevent the patients with CKD from progression into ESRD.
Ε2 allele might affect the susceptibility of ESRD through the lipid and non-lipid mediated mechanisms. First, apoE is mainly secreted by the mesangial cells in kidney [2]. Nonlipid-mediated pathways may be involved in the direct effect on kidney remodeling by apoE [32]. Through induction of matrix heparin sulfate proteoglycan (HSPG), the proliferation of mesangial cell could be differentially inhibited by the apoE’s isoforms [34]. Ε2 shows the least antiproliferative effect on the mesangial cell. More severe histological damage was found among patients with ε2 allele and IgA nephropathy [35]. Additionally, apoE may have isoform-specific effects on vascular smooth muscle proliferation, which may affect progression of ESRD [36]. Second, lipid disorder could accelerate the progression to ESRD. ApoE plays an important role in the modulation of circulating lipid and lipoprotein. The structural basis of apoE protein types are determined by the apoE polymorphism [37]. ApoE2 which is produced by the ε2 allele has the lowest binding ability to the receptor. Then the uptaking and clearance of CM or VLDL remnants were impaired in the liver [5]. The ε2 carriers had higher VLDL, TG and apoE2 concentration in the serum, which is associated with type iii hyperlipidemia and kidney disease [38]. The performance the hypertriglyceridemia caused by apoE2 might easily lead to renal vascular atherosclerosis which promote the development of ESRD.
In the results of subgroup analysis by ethnicity, there was a significant association in East Asians, but not in Caucasians. The findings from races seemed different. The possible reasons included: (1) apoE allele frequencies were affected by different genetic backgrounds and geographical diversities; (2) different eating habits may lead to various types of lipid metabolism; (3) the primary kidney disease of ESRD was different, in which the apoE allele played different roles. Although the P value of ε2 on ESRD risk in Caucasians was 0.03, a positive association between ε2 and ESRD risk may exist. When studies in Caucasians got larger sample size, we might have sufficient statistical power to detect the slight effect.
Both cumulative meta-analysis and one-way sensitivity analysis got highly stable results which were in accordance with the primary result of ε2. Publication bias was little which made the results more reliable. In overall populations, moderate heterogeneity was observed for the ε2ε3, ε3ε3, ε3ε4, ε3 and ε4 polymorphisms. Subgroup analysis was used to find the sources of heterogeneity. In the subgroup analysis by ethnicity, the heterogeneity among the comparisons decreased effectively or disappeared. So the main source of heterogeneity might be from different populations. The sensitivity analysis based on studies in HWE [14,15,19-22,24,26-31] found the same results with the ones including all the studies [14,15,18-31]. Different methods for meta-analysis all suggested that ε2 allele might play a role in the etiology of ESRD.
There were some limitations of this meta-analysis. First of all, the number of available studies included in this meta-analysis was moderate. Only one study provided the apoE gene frequencies in different etiologies of ESRD [14], and the subgroup analysis by etiology was not able to perform, especially about the influences of diabetic nephropathy and non-diabetic nephropathies. More studies not only on all-cause ESRD but also on ESRD with or without diabetic nephropathy could solve this problem in the future. Second, Asians and Caucasians were studied in most of the case-control studies, so the results may be applicable only to these ethnicities. Third, gene-gene and gene-environment interactions could not be addressed in this study because of insufficient data from published studies. Finally, except for the selected databases we have searched, there may be some relevant studies with negative results were missed.
Conclusion
In conclusion, the ε2 allele of apoE gene might increase the risk of ESRD, and expressed more apoE protein. More well-designed studies are needed to prove these associations in the future.
Supporting Information
PRISMA 2009 Flow Diagram.
(TIF)
PRISMA 2009 Checklist.
(DOC)
Funding Statement
The work was supported by Pujiang Talent Program of Shanghai Municipality (12PJ1403300) and Major Basic Research Project of Science and Technology Commission of Shanghai Municipality (12DJ1400300). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Freedman BI, Satko SG (2000) Genes and renal disease. Curr Opin Nephrol Hypertens 9: 273-277. doi: 10.1097/00041552-200005000-00011. PubMed: 10847329. [DOI] [PubMed] [Google Scholar]
- 2. Blue ML, Williams DL, Zucker S, Khan SA, Blum CB (1983) Apolipoprotein E synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A 80: 283-287. doi: 10.1073/pnas.80.1.283. PubMed: 6572003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karavia EA, Papachristou DJ, Kotsikogianni I, Giopanou I, Kypreos KE (2011) Deficiency in apolipoprotein E has a protective effect on diet-induced nonalcoholic fatty liver disease in mice. FEBS J 278: 3119-3129. doi: 10.1111/j.1742-4658.2011.08238.x. PubMed: 21740524. [DOI] [PubMed] [Google Scholar]
- 4. Li HF, Han CF, Wang YX, Lu YS, Zou HQ et al. (2010) Effect of apolipoprotein E gene polymorphism on serum lipid level before and after renal transplantation. Transplant Proc 42: 2513-2517. doi: 10.1016/j.transproceed.2010.04.023. PubMed: 20832534. [DOI] [PubMed] [Google Scholar]
- 5. Eto M, Saito M, Okada M, Kume Y, Kawasaki F et al. (2002) Apolipoprotein E genetic polymorphism, remnant lipoproteins, and nephropathy in type 2 diabetic patients. Am J Kidney Dis 40: 243-251. doi: 10.1053/ajkd.2002.34502. PubMed: 12148096. [DOI] [PubMed] [Google Scholar]
- 6. Srinivasan SR, Radhakrishnamurthy B, Vijayagopal P, Berenson GS (1991) Proteoglycans, lipoproteins, and atherosclerosis. Adv Exp Med Biol 285: 373-381. PubMed: 1858569. [DOI] [PubMed] [Google Scholar]
- 7. Saito T, Sato H, Oikawa S, Kudo K, Kurihara I et al. (1993) Lipoprotein glomerulopathy. Report of a normolipidemic case and review of the literature. Am J Nephrol 13: 64-68. doi: 10.1159/000168591. PubMed: 8322843. [DOI] [PubMed] [Google Scholar]
- 8. De Feo E, Cefalo C, Arzani D, Amore R, Landolfi R et al. (2012) A case-control study on the effects of the apolipoprotein E genotypes in nonalcoholic fatty liver disease. Mol Biol Rep 39: 7381-7388. doi: 10.1007/s11033-012-1570-7. PubMed: 22350157. [DOI] [PubMed] [Google Scholar]
- 9. Weisgraber KH (1994) Apolipoprotein E: structure-function relationships. Adv Protein Chem 45: 249-302. doi: 10.1016/S0065-3233(08)60642-7. PubMed: 8154371. [DOI] [PubMed] [Google Scholar]
- 10. Zhou TB, Qin YH, Xu HL (2012) Association of apoE gene expression and its gene polymorphism with nephrotic syndrome susceptibility: a meta-analysis of experimental and human studies. Mol Biol Rep 39: 9347-9354. doi: 10.1007/s11033-012-1751-4. PubMed: 22760259. [DOI] [PubMed] [Google Scholar]
- 11. Araki S, Moczulski DK, Hanna L, Scott LJ, Warram JH et al. (2000) APOE polymorphisms and the development of diabetic nephropathy in type 1 diabetes: results of case-control and family-based studies. Diabetes 49: 2190-2195. doi: 10.2337/diabetes.49.12.2190. PubMed: 11118024. [DOI] [PubMed] [Google Scholar]
- 12. Werle E, Fiehn W, Hasslacher C (1998) Apolipoprotein E polymorphism and renal function in German type 1 and type 2 diabetic patients. Diabetes Care 21: 994-998. doi: 10.2337/diacare.21.6.994. PubMed: 9614620. [DOI] [PubMed] [Google Scholar]
- 13. Hubacek JA, Bloudickova S, Kubinova R, Pikhart H, Viklicky O et al. (2009) Apolipoprotein E polymorphism in hemodialyzed patients and healthy controls. Biochem Genet 47: 688-693. doi: 10.1007/s10528-009-9266-y. PubMed: 19565203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roussos L, Ekström U, Ehle PN, Oqvist B, Floren CH (2004) Apolipoprotein E polymorphism in 385 patients on renal replacement therapy in Sweden. Scand J Urol Nephrol 38: 504-510. doi: 10.1080/00365590410033443. PubMed: 15841787. [DOI] [PubMed] [Google Scholar]
- 15. Feussner G, Wey S, Bommer J, Deppermann D, Grützmacher P et al. (1992) Apolipoprotein E phenotypes and hyperlipidemia in patients under maintenance hemodialysis. Hum Genet 88: 307-312. PubMed: 1733833. [DOI] [PubMed] [Google Scholar]
- 16. Nie W, Xue C, Chen J, Xiu Q (2013) Secretoglobin 1A member 1 (SCGB1A1) +38A/G polymorphism is associated with asthma risk: A meta-analysis. Gene, 528: 304–8. PubMed: 23820082. [DOI] [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634. doi: 10.1136/bmj.315.7109.629. PubMed: 9310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eggertsen G, Heimbürger O, Stenvinkel P, Berglund L (1997) Influence of variation at the apolipoprotein E locus on lipid and lipoprotein levels in CAPD patients. Nephrol Dial Transplant 12: 141-144. doi: 10.1093/ndt/12.1.141. PubMed: 9027789. [DOI] [PubMed] [Google Scholar]
- 19. Gejyo F, Kimura H, Suzuki S, Miyazaki R, Naiki H et al. (1997) Apolipoprotein E and alpha 1-antichymotrypsin in dialysis-related amyloidosis. Kidney Int Suppl 62: S75-S78. PubMed: 9350687. [PubMed] [Google Scholar]
- 20. Olmer M, Renucci JE, Planells R, Bouchouareb D, Purgus R (1997) Preliminary evidence for a role of apolipoprotein E alleles in identifying haemodialysis patients at high vascular risk. Nephrol Dial Transplant 12: 691-693. doi: 10.1093/ndt/12.4.691. PubMed: 9140995. [DOI] [PubMed] [Google Scholar]
- 21. Kohlmeier M, Saupe J, Schaefer K, Asmus G (1998) Bone fracture history and prospective bone fracture risk of hemodialysis patients are related to apolipoprotein E genotype. Calcif Tissue Int 62: 278-281. doi: 10.1007/s002239900430. PubMed: 9501964. [DOI] [PubMed] [Google Scholar]
- 22. Choi KH, Song HY, Shin SK, Noh H, Kang SW et al. (1999) Influence of apolipoprotein E genotype on lipid and lipoprotein levels in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 15: 243-246. PubMed: 10682111. [PubMed] [Google Scholar]
- 23. Imura T, Kimura H, Gejyo F (1999) Apolipoprotein E phenotypes in hemodialysis patients. Kidney Int Suppl 71: S245-S247. PubMed: 10412789. [DOI] [PubMed] [Google Scholar]
- 24. Oda H, Yorioka N, Ueda C, Kushihata S, Yamakido M (1999) Apolipoprotein E polymorphism and renal disease. Kidney Int Suppl 71: S25-S27. PubMed: 10412731. [DOI] [PubMed] [Google Scholar]
- 25. Güz G, Nurhan Ozdemir F, Sezer S, Işiklar I, Arat Z et al. (2000) Effect of apolipoprotein E polymorphism on serum lipid, lipoproteins, and atherosclerosis in hemodialysis patients. Am J Kidney Dis 36: 826-836. doi: 10.1053/ajkd.2000.17682. PubMed: 11007687. [DOI] [PubMed] [Google Scholar]
- 26. Zahalkova J, Vaverkova H, Novotny D, Kosatikova Z (2002) Impaired triglyceride tolerance in hemodialysis patients with different apolipoprotein E (apo E) isoforms. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 146: 73-76 [DOI] [PubMed]
- 27. Asakimori Y, Yorioka N, Tanaka J, Kohno N (2003) Effect of polymorphism of the endothelial nitric oxide synthase and apolipoprotein E genes on carotid atherosclerosis in hemodialysis patients. Am J Kidney Dis 41: 822-832. doi: 10.1016/S0272-6386(03)00030-1. PubMed: 12666069. [DOI] [PubMed] [Google Scholar]
- 28. Liberopoulos E, Siamopoulos K, Elisaf M (2004) Apolipoprotein E and renal disease. Am J Kidney Dis 43: 223-233. doi: 10.1053/j.ajkd.2003.10.013. PubMed: 14750087. [DOI] [PubMed] [Google Scholar]
- 29. Huang ZH, Wu XF (2005) Study on apolipoprotein E polymorphism in patients with end stage renal failure. Chongqing Medcine 34: 1455-1458. [Google Scholar]
- 30. Arikan H, Koc M, Sari H, Tuglular S, Ozener C et al. (2007) Associations between apolipoprotein E gene polymorphism and plasminogen activator inhibitor-1 and atherogenic lipid profile in dialysis patients. Ren Fail 29: 713-719. doi: 10.1080/08860220701460129. PubMed: 17763167. [DOI] [PubMed] [Google Scholar]
- 31. Al-Muhanna F, Al-Mueilo S, Al-Ali A, Larbi E, Rubaish A et al. (2008) Polymorphism in methylenetetrahydrofolate reductase, plasminogen activator inhibitor-1, and apolipoprotein E in hemodialysis patients. Saudi J Kidney Dis Transpl 19: 937-941. PubMed: 18974580. [PubMed] [Google Scholar]
- 32. Hsu CC, Kao WH, Coresh J, Pankow JS, Marsh-Manzi J et al. (2005) Apolipoprotein E and progression of chronic kidney disease. JAMA 293: 2892-2899. doi: 10.1001/jama.293.23.2892. PubMed: 15956634. [DOI] [PubMed] [Google Scholar]
- 33. Chu AY, Parekh RS, Astor BC, Coresh J, Berthier-Schaad Y et al. (2009) Association of APOE polymorphism with chronic kidney disease in a nationally representative sample: a Third National Health and Nutrition Examination Survey (NHANES III) Genetic Study. BMC Med Genet 10: 108. doi: 10.1186/1471-2350-10-108. PubMed: 19852818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen G, Paka L, Kako Y, Singhal P, Duan W et al. (2001) A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J Biol Chem 276: 49142-49147. doi: 10.1074/jbc.M104879200. PubMed: 11579084. [DOI] [PubMed] [Google Scholar]
- 35. Yorioka N, Nishida Y, Oda H, Watanabe T, Yamakido M (1999) Apolipoprotein E polymorphism in IgA nephropathy. Nephron 83: 246-249. doi: 10.1159/000045517. PubMed: 10529631. [DOI] [PubMed] [Google Scholar]
- 36. Ali K, Lund-Katz S, Lawson J, Phillips MC, Rader DJ (2008) Structure-function properties of the apoE-dependent COX-2 pathway in vascular smooth muscle cells. Atherosclerosis 196: 201-209. doi: 10.1016/j.atherosclerosis.2007.03.038. PubMed: 17531997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshida T, Kato K, Fujimaki T, Yokoi K, Oguri M et al. (2009) Association of a polymorphism of the apolipoprotein E gene with chronic kidney disease in Japanese individuals with metabolic syndrome. Genomics 93: 221-226. doi: 10.1016/j.ygeno.2008.11.001. PubMed: 19056482. [DOI] [PubMed] [Google Scholar]
- 38. Sakatsume M, Kadomura M, Sakata I, Imai N, Kondo D et al. (2001) Novel glomerular lipoprotein deposits associated with apolipoprotein E2 homozygosity. Kidney Int 59: 1911-1918. doi: 10.1046/j.1523-1755.2001.0590051911.x. PubMed: 11318963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Flow Diagram.
(TIF)
PRISMA 2009 Checklist.
(DOC)