Abstract
We examined the efficacy of using attenuated non-replicating Toxoplasma gondii uracil auxotrophs that can be safely delivered as anticancer immunotherapeutics. This strategy exerted remarkable therapeutic activity in murine models of melanoma and ovarian carcinoma, and holds broad potential for the development of novel, highly effective anticancer vaccines.
Keywords: anticancer vaccines, cancer immunotherapy, CD8+ T cells, non-replicating Toxoplasma gondii, reversing tumor-derived immunosuppression
Tumors promote their own growth and evade immunosurveillance. The ability of malignant cells to establish immunosuppressive conditions plays a major role in tumor progression by interfering with immunological control mechanisms. The precise nature of tumor-associated immunosuppression varies with cancer type, is biologically complex, and often involves perturbation of both innate and adaptive mechanisms that would otherwise eradicate malignant lesions.1
While the use of pathogenic microorganisms in cancer immunotherapy is not novel, protozoan parasites are relatively unexplored in this context. Toxoplasma gondii is an obligate intracellular protozoan parasite that actively invades host cells. Toxoplasma cells preferentially contact and invade myeloid cells including dendritic cells and monocytes/macrophages,2 which are frequently involved in tumor-elicited immunosuppression. By invading myeloid cells, T. gondii gains direct access to the control of innate immune cells, generally resulting in the elicitation of potent TH1 immune responses.3 During invasion, Toxoplasma cells secrete a repertoire of specialized molecules that function to seize control of the host cell from within.3 The parasite also hijacks bystander cells as the molecules that it produces are injected into cells that are contacted but are not invaded.4 For example, T. gondii injects the rhoptry (ROP)16 kinase into macrophages, suppressing the signal transducer and activator of transcription 3 (STAT)3-dependent production of interleukin-12 (IL-12), and upregulating arginase 1 upon the activation of STAT6.5 Toxoplasma cells also secrete ROP18, a kinase that protects the vacuole housing intracellular parasites from innate immune attack mechanisms mediated by a family of interferon γ (IFNγ)-activated GTPases.6 Additional molecules secreted by T. gondii play significant roles in manipulating host cells and immune responses.3
A safe, live-attenuated, non-replicating variant of T. gondii was created as an uracil auxotroph (cps) strain.7 While uracil auxotrophs normally invade mammalian cells, they do not replicate in the absence of uracil, thus exhibiting an exquisite degree of attenuation of virulence in both normal and severely immunodeficient mice.7,8 In contrast to many prokaryotic microbes, the eukaryotic T. gondii does not harbor any significant toxin or otherwise toxic molecule. We therefore reasoned that strongly polarized TH1 host responses driven T. gondii-secreted factors and the manipulation of innate immune cells by means of the cps strain would stimulate responses in the tumor microenvironment that could break tumor-associated immunosuppression.
Immature CD11c+ dendritic cells accumulate in high amounts within solid epithelial tumors including ovarian carcinomas, and deliver signals that create a highly immunosuppressive microenvironment.9 Treatment of established aggressive vascular endothelial growth factor (VEGF)-expressing ID8 ovarian tumors with the cps strain resulted in tumor regression and improved the survival of tumor-bearing mice.9 Of note, the administration of the cps strain was equally effective in naïve mice as well as in mice that were immune to Toxoplasma. The immunotherapeutic effects of cps cells was completely dependent on IL-12, but not on Toll-like receptor (TLR) adaptor myeloid differentiation 88 (MYD88).9 In the tumor microenvironment as well as ex vivo, the cps strain preferentially invaded CD45+CD11c+ cells and both cps-infected and cps-contacted cells exhibited increased expression levels of the co-stimulatory molecules CD80 and CD86. The treatment of ovarian carcinomas with the cps strain rapidly reversed tumor-associated immunosuppression and stimulated the priming of CD8+ T-cell responses by antigen-presenting cells.9 Tumor antigen-specific CD8+ (and granzyme B+) T cells were increased both in the spleen and in the tumor microenvironment upon the administration of the cps strain,9 and adoptive transfer experiments demonstrated that T cells from treated mice potently suppressed the development of ovarian carcinomas.9 The cps strain also stimulated the recruitment of numerous cell types to neoplastic lesions and to the spleen. Of note, while the TH17+ cells were not increased by our immunotherapeutic approach, the percentage of intratumoral regulatory T cells (CD4+FOXP3+ T cells) was significantly decreased.
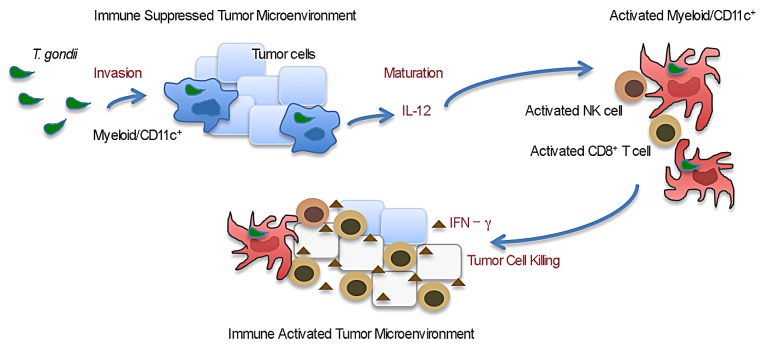
Along similar lines, the administration of the cps strain elicited the immune system-mediated regression of established B16F10 melanomas.10 More than 90% of cps-treated mice survived B16F10 melanoma and most of these animals developed localized and/or systemic vitiligo, as indication of the recognition of melanocytes by the immune system. The therapeutic efficacy of cps cells required the participation of both natural killer (NK) cells and CD8+ T lymphocytes but not of CD4+ T cells. Moreover, also in this setting, the efficacy of cps-based immunotherapy was completely dependent on IL-12 and IFNγ. Living cps parasites were necessary for the elicitation of antitumor responses, suggesting a requirement for the active invasion of host cells by the parasite and their manipulation upon the secretion of effector molecules. Multiple cell types were invaded by cps parasites in the melanoma microenvironment, and various cell types were recruited to neoplastic lesions, tumor-draining lymph node, and the spleen.10 The treatment increased the frequency of IFNγ-expressing CD8+ T cells specific for a melanoma-associated antigen, namely dopachrome tautomerase (DCT, also known as TRP2). The re-challenge of mice that survived melanoma upon the administration of the cps strain with living melanoma cells failed to support a second wave of oncogenesis, suggesting that cps-based immunotherapy generated significant memory responses.10 Collectively, these results reveal that immunotherapeutic approaches based on a non-replicating variant of T. gondii can reverse tumor-associated immunosuppression and stimulate effective immune responses against solid tumors (Fig. 1).
Figure 1. Active invasion by Toxoplasma gondii reverses tumor-elicited immunosuppression and activates tumor-targeting immune responses. Immunosuppressive myeloid CD11c+ cells in the tumor microenvironment are preferentially invaded by the non-replicating cps strain of T. gondii. Invaded or contacted myeloid cells are activated to produce interleukin-12 (IL-12) and to express increased levels of the co-stimulatory molecules CD80 and CD86 on their surface. The cps strain induces the maturation of myeloid CD11c+ cells, leading to increased antigen-presentation and T-cell priming. Eventually this results in the expansion of activated natural killer (NK) cells and CD8+ T lymphocytes, which release interferon γ (IFNγ) into the tumor microenvironment. Cellular responses such as those mediated by NK cells and tumor-specific CD8+ T lymphocytes mediate the killing of cancer, hence causing tumor regression.
A major advantage of cps-based immunotherapy is its versatility. The cps strain was originally developed as a self-adjuvant platform for stimulating potent TH1 immune responses to engineered CD8+ T-cell vaccines.7 T. gondii uracil auxotrophs can be easily engineered with conventional genetic techniques to exacerbate vaccine-elicited immune responses, to express specific molecules (or exert selected functions) in the tumor microenvironment, or to selectively target particular cell types. These versatile biological features along with the inherent and potent immunotherapeutic potential of the cps platform itself open multiple avenues and a wide-range of potential applications. Exploiting the unique biology of the safe Toxoplasma uracil auxotroph vaccine platform is expected to drive the development of innovative cancer vaccines that are able to eradicate established lesions as well as prevent disease recurrence.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grants.
Citation: Fox B, Sanders K, Bzik D. Non-replicating Toxoplasma gondii reverses tumor-associated immunosuppression. OncoImmunology 2013; 2:e26296; 10.4161/onci.26296
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26296
References
- 1.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun. 2005;73:703–11. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denkers EY, Bzik DJ, Fox BA, Butcher BA. An Inside Job: Hacking Into JAK/STAT signaling Cascades by the Intracellular Protozoan Toxoplasma gondii. Infect Immun. 2012;80:476–82. doi: 10.1128/IAI.05974-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koshy AA, Dietrich HK, Christian DA, Melehani JH, Shastri AJ, Hunter CA, Boothroyd JC. Toxoplasma co-opts host cells it does not invade. PLoS Pathog. 2012;8:e1002825. doi: 10.1371/journal.ppat.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, Herbert DR, Bzik DJ, Denkers EY. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011;7:e1002236. doi: 10.1371/journal.ppat.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, et al. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe. 2010;8:484–95. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox BA, Bzik DJ. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature. 2002;415:926–9. doi: 10.1038/415926a. [DOI] [PubMed] [Google Scholar]
- 8.Fox BA, Bzik DJ. Avirulent uracil auxotrophs based on disruption of orotidine-5′-monophosphate decarboxylase elicit protective immunity to Toxoplasma gondii. Infect Immun. 2010;78:3744–52. doi: 10.1128/IAI.00287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird JR, Fox BA, Sanders KL, Lizotte PH, Cubillos-Ruiz JR, Scarlett UK, Rutkowski MR, Conejo-Garcia JR, Fiering S, Bzik DJ. Avirulent Toxoplasma gondii generates therapeutic antitumor immunity by reversing immunosuppression in the ovarian cancer microenvironment. Cancer Res. 2013;73:3842–51. doi: 10.1158/0008-5472.CAN-12-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird JR, Byrne KT, Lizotte PH, Toraya-Brown S, Scarlett UK, Alexander MP, Sheen MR, Fox BA, Bzik DJ, Bosenberg M, et al. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol. 2013;190:469–78. doi: 10.4049/jimmunol.1201209. [DOI] [PMC free article] [PubMed] [Google Scholar]