Abstract
We have recently discovered that mast cells promote pancreatic tumorigenesis by exacerbating the cellular and extracellular signaling in the desmoplastic microenvironment. Our findings demonstrate for the first time that targeting mast cells can block the progression of pancreatic neoplasms and prolong the survival of tumor-bearing immunocompetent hosts.
Keywords: mast cells, pancreatic cancer, pancreatic stellate cells, tumor microenvironment
Background
Pancreatic cancer has an extremely poor prognosis and is indeed the fourth leading cause of cancer-related deaths in the United States. The 5-y survival rate of pancreatic cancer patients is approximately 6%, and the median survival time upon diagnosis is 6 mos. Over the past several decades, these statistics have improved by very little. Pancreatic ductal adenocarcinoma (PDAC) is the most common histological type of pancreatic cancer, accounting for over 95% of cases and mortality.1 Pancreatic cancer lesions include not only aggressively growing malignant ducts, but also a dense stromal microenvironment, termed “desmoplasia,” which fosters tumorigenesis and the inherent resistance of this neoplasm to almost all treatments, including chemotherapy (be it conventional or targeted), radiotherapy and immunotherapy.
The microenvironment of pancreatic cancer comprises neoplastic cells, a non-malignant cellular component as well as conspicuous deposits of extracellular matrix. Pancreatic stellate cells (PSCs, akin to cancer-associated fibroblasts, CAFs)2 and immune cells3 are the most prominent non-malignant cells of the pancreatic tumor microenvironment. In normal conditions, PSCs exist in a quiescent state, sparsely disseminated around pancreatic ducts and acini, and operate as stores of vitamin A droplets. Upon inflammation, injury or hitherto unidentified stimuli, PSCs are activated to proliferate in an uncontrolled manner, leading to fibrosis. PSCs have been recognized as a pancreas-specific mesenchymal cell compartment that plays a key role in the fibrotic stroma, forming up to 90% of the tumor volume, a property that is unique to pancreatic cancer.4 Immune cells that infiltrate the pancreatic microenvironment include components of both the innate and adaptive immune systems, such as macrophages, dendritic cells, lymphocytes and mast cells.5 Mast cells, multifaceted tissue-homing secretory cells, have been intensively investigated during the past century for their contribution to fibrotic responses in the course of chronic inflammation as well as for their central role in allergic and anaphylactic conditions. Recently, mast cells have attributed a key role in tumor progression, as their intratumoral abundance has been shown to correlate with improved or worsened prognosis in patients affected by a variety of malignancies.6 Mast cells tend to cluster at the peripheral invasive edges of pancreatic cancers, and three reports indicate that high amounts of these cells correlate with worse prognosis in this setting.7-9 However, the exact mechanism whereby mast cells contribute to the progression of pancreatic cancer is complex and far from being defined. Moreover, whether inhibiting the activity of mast cells or preventing their infiltration into neoplastic lesions can affect the outcome of pancreatic cancer remains an open conundrum. We hypothesized that mast cells promote the growth of both PSCs and pancreatic cancer cells, thus contributing to tumor progression as well as to the establishment of a pronounced desmoplastic microenvironment.
Mast Cells are Essential for the Desmoplastic Microenvironment of Pancreatic Cancer
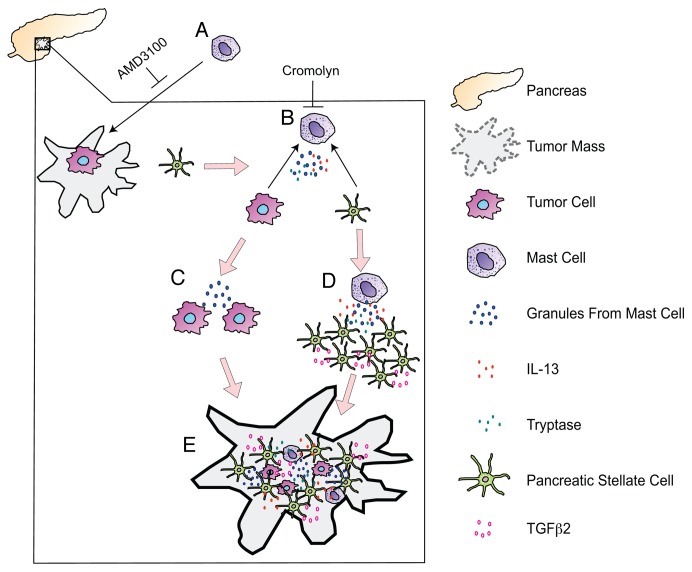
To examine the dynamics of mast cells in pancreatic cancer and understand the mutual relationships between mast cells, malignant cells and PSCs, we established an in vitro co-culture system.10 Because high amounts of mast cells are found in pancreatic carcinomas,7-9 but few of these cells infiltrate the normal pancreas, we first wished to determine whether pancreatic cancer cells or PSCs stimulate the recruitment of mast cells to the tumor microenvironment. We found that pancreatic cancer cells, but not PSCs, induce mast cell migration. Our findings suggest that both malignant cells and PSCs promote the degranulation of tumor-infiltrating mast cells and stimulate them to secrete cytokines. In turn, activated mast cells favor the proliferation of pancreatic cancer cells and PSCs. In particular, we observed that mast cell-derived cytokines including interleukin-13 (IL-13) and tryptase drive the proliferation of stromal cells. The secretion of transforming growth factor β2 (TGFβ2) by PSCs was also upregulated, in turn activating a SMAD2-dependent signal transduction cascade that accelerates the deposition of extracellular matrix components and fibrosis. Thus, malignant pancreatic cells promote the recruitment of mast cells to the tumor microenvironment. In this setting, both malignant cells and PSCs activate mast cells to release cytokines that stimulate their own proliferation. Such an interaction between pancreatic cancer cells, PSCs and mast cell eventually culminates in a feedforward circuitry leading to tumor progression (Fig. 1). These findings establish a new paradigm in pancreatic cancer, namely, that mast cells promote pancreatic tumorigenesis by exacerbating the cellular and extracellular signaling in the desmoplastic microenvironment.
Figure 1. Interactions between mast cells, pancreatic stellate cells, and malignant cells promote the desmoplastic pancreatic cancer microenvironment. (A) Pancreatic cancer cells promote the recruitment of mast cells to neoplastic lesions, which can be blocked by interfering with the chemokine (C-X-C motif) receptor 4 (CXCR4) signaling axis. (B) Malignant cells and pancreatic stellate cells (PSCs) stimulate mast cell activation, which can be prevented by the mast cell stabilizer cromolyn. (C) Activated mast cells support tumor growth. (D) Upon activation, mast cell-derived cytokines such as interleukin-13 (IL-13) and tryptase promote PSCs to proliferate and secrete transforming growth factor β2 (TGFβ2). In (C and D), a feedforward loop that may serve to accelerate the effects of mast cells on the tumor microenvironment is depicted. (E) We suggest that tumor-infiltrating mast cells, by promoting the proliferation of both malignant cells and PSCs, favor the progression of pancreatic cancer and contribute to its desmoplastic microenvironment.
Prospective on Developing Mast Cell-Targeting Anticancer Therapies
Following the results obtained with our in vitro co-culture system, we hypothesized that inhibiting the migration of mast cells to neoplastic lesions and/or blocking their activation in situ might affect the growth of pancreatic cancer in vivo. To address this hypothesis, we took advantage of an orthotopic PDAC mouse model. Indeed, blocking the migration of mast cells with the chemokine (C-X-C motif) receptor 4 (CXCR4) antagonist AMD3100 or inhibiting mast cell activation with the well-known mast cell stabilizer cromolyn, disrupted the reinforcing interactions between malignant cells, PSCs and mast cells. The significant tumor shrinkage observed in cromolyn-treated tumor-bearing mice and the prolonged survival of AMD3100-treated tumor-bearing animals indicate that blocking mast cell function in the tumor microenvironment may be a key intervention to interrupt pancreatic cancer progression. Our findings demonstrate that mast cells contribute to the desmoplastic microenvironment of pancreatic cancer by promoting the proliferation of PSCs, and suggest that targeting mast cells can block disease progression and improve the survival of immunocompetent hosts affected by this neoplasm.10 Manipulating the migration and activation of mast cells might therefore be translated to the clinic in a neoadjuvant setting, to increase the survival of pancreatic cancer patients.
Citation: Ma Y, Ullrich SE. Intratumoral mast cells promote the growth of pancreatic cancer. OncoImmunology 2013; 2:e 25913; 10.4161/onci.25913
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25964
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 5.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230–40. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribatti D, Crivellato E. The controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol. 2009;275:89–131. doi: 10.1016/S1937-6448(09)75004-X. [DOI] [PubMed] [Google Scholar]
- 7.Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu Y, Abbruzzese JL, Liu YJ, Logsdon CD, Hwu P. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:7015–23. doi: 10.1158/1078-0432.CCR-11-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG, Dangi-Garimella S, Wang E, Munshi HG, Khazaie K, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res. 2010;16:2257–65. doi: 10.1158/1078-0432.CCR-09-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai SW, Yang SZ, Gao J, Pan K, Chen JY, Wang YL, Wei LX, Dong JH. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery. 2011;149:576–84. doi: 10.1016/j.surg.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Hwang RF, Logsdon CD, Ullrich SE. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013;73:3927–37. doi: 10.1158/0008-5472.CAN-12-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]