Abstract
Anticancer immunotherapy is a promising treatment modality since it bears the potential of being highly specific, but effective clinical applications are still under development. We have recently described an exosome-based strategy for co-delivery of α-galactosylceramide and a tumor-associated antigen that synergistically potentiates tumor-specific adaptive immune responses while preventing the anergy of invariant natural killer T (iNKT) cells. We propose that the next generation of exosome-based immunotherapies should involve iNKT-cell ligands to induce a broad, amplified and sustainable antitumor immune response.
Keywords: bone marrow-derived dendritic cells, exosomes, invariant natural killer T cells, ovalbumin, α-galactosylceramide
Anticancer immunotherapeutic regimens must activate multiple effectors of the innate and the adaptive immune system in order to efficiently counteract the escape mechanisms set in place by malignant cells. The cellular components of the immune system that are important for treatment outcome have been shown to vary, at least to some degree, with tumor type and treatment modality. Thus, while natural killer (NK) cells are needed for so-called “missing self”-based cellular cytotoxicity (resulting in the elimination of cells that do not express MHC class I molecules), γδ T lymphocytes eliminate cells that express altered self antigens, CD4+ T lymphocytes help B cells to produce antibodies that are necessary for antibody-dependent cellular cytotoxicity (ADCC), and CD8+ T lymphocytes exert direct, tumor-specific cytotoxic functions.1 Invariant natural killer T (iNKT) cells are important in that they integrate the activation of the innate and adaptive immune systems by secreting high amounts of immunostimulatory cytokines and cross-licensing antigen-presenting cells (APCs) such as dendritic cells (DCs).2
Exosomes are nanovesicles originating from the endosomal compartment that can carry immunostimulatory or immunosuppressive molecules, and are currently explored as therapeutic vehicles. We have recently shown that DC-derived exosomes loaded with the iNKT-cell ligand α-galactosylceramide (αGC) and the model antigen ovalbumin (OVA) induce strong innate and OVA-specific adaptive immune responses while preventing the anergy of iNKT cells.3 In particular, we demonstrated that the intravenous administration of αGC/OVA-loaded exosomes leads to a strong, sequential activation of iNKT cells, NK cells, γδ T cells, CD4+ T cells as well as OVA-specific CD8+ T and B lymphocytes. Moreover, we found that exosomes are more potent in inducing γδ T cell-dependent and OVA-specific T and B cell-mediated immunity than comparable amounts of soluble αGC and OVA. In contrast, soluble αGC induces more robust iNKT- and NK-cell responses than αGC-loaded exosomes, indicating that different mechanisms are responsible for the activation of cells of the innate and adaptive immune systems.
Despite the potent adjuvant activity of iNKT cells, their use in clinical settings has been limited since a single injection of soluble αGC is sufficient to render iNKT cells unresponsive (anergy).4,5 Conversely, it has previously been documented that nanoparticle-formulated αGC results in prolonged iNKT-cell responsiveness, even upon several injections.6 Similarly, an antigen-CD1d-αGC fusion protein has been shown to prevent the induction of iNKT-cell anergy.7 Moreover, the co-delivery of αGC with microbial or synthetic glycolipid antigens on latex beads reportedly increases its adjuvant activity through the iNKT cell-dependent cross-activation of APCs, resulting in the release of T cell-attracting chemokines.8 Our findings suggest that αGC- and OVA-loaded exosomes boost T- and B-cell immunity by a similar mechanism. We propose that an early activation of iNKT cells in the spleen leads to efficient antigen presentation by DCs and hence improved T- and B-cell responses (Fig. 1). We observed that the levels of circulating interferon γ (IFNγ) levels and OVA-specific antibodies are significantly increased upon the injection of αGC/OVA-loaded exosomes as compared with that of soluble ligands. Based on our results and the aforementioned studies, we speculate that the delivery of both αGC and antigens to the same APC is crucial for the activation of antigen-specific T cells, thus having a critical impact on the outcome of immune responses. We also demonstrated that a single dose of αGC/OVA-loaded exosomes not only reduces the growth of established OVA-expressing B16 melanomas but also increases the survival of mice bearing these neoplasms. Importantly, a second dose of αGC/OVA-loaded exosomes significantly increased tumor infiltration by OVA-specific CD8+ T cells as well as the titers of OVA-specific antibodies, hence further prolonging the survival of tumor-bearing mice.
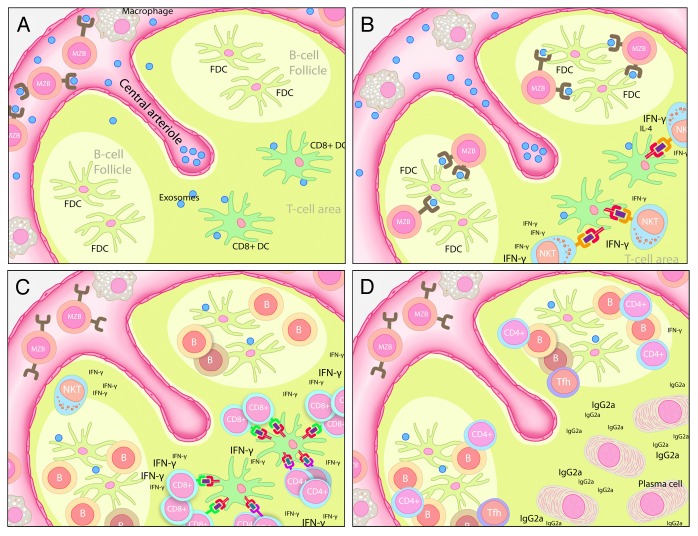
Figure 1. Possible mechanisms for the sequential activation of immune cells by exosomes. (A) Exosomes loaded with α-galactosylceramide (αGC) and tumor-associated antigens are injected intravenously and taken up by splenic marginal zone B cells (MZBs) in the B cell zone and dendritic cells (DCs), which migrate into the T-cell zone. (B) MZBs deposit antigen on follicular DCs (FDCs) in the splenic B-cell zone, while DCs mediate lipid antigen presentation to iNKT cells in the T-cell zone (C) CD8+ DCs become cross-licensed to efficiently present peptide antigens to CD4+ and CD8+ T cells, while antigen-specific B cells become activated upon interaction with antigen-presenting FDCs. (D) Activated CD4+ T cells home to the B-cell/T-cell border and assist B-cell activation, thus favoring isotype switching and the production of antigen-specific antibodies.
In summary, exosomes might provide a new delivery platform for antigens and αGC that optimally harnesses the adjuvant activity of iNKT cells. In addition to αGC and antigens, DC-derived exosomes contain various immunostimulatory molecules, including heat-shock proteins, the α chain of the interleukin-15 receptor (IL15Rα), killer cell lectin-like receptor subfamily K, member 1 (KLRK1) ligands and Toll-like receptor (TLR) agonists, and might be further modified to increase their immunostimulatory efficacy.9 The design of exosomes might therefore be adjusted depending on the desired immunological outcome. We propose that the next step in exosome-induced immune responses to be explored in clinical trials should include iNKT cell ligands for the induction of broad immune responses that engage both the innate and the adaptive arms of the immune system.
Disclosure of Potential Conflicts of Interest
The authors declare that there are no conflicts of interest.
Citation: Gehrmann U, Hiltbrunner S, Näslund T, Gabrielsson S. Potentiating antitumor immunity with αGC-loaded exosomes. OncoImmunology 2013; 2:e26261; 10.4161/onci.26261
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26261
References
- 1.Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–8. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehrmann U, Hiltbrunner S, Georgoudaki AM, Karlsson MC, Näslund TI, Gabrielsson S. Synergistic induction of adaptive antitumor immunity by codelivery of antigen with α-galactosylceramide on exosomes. Cancer Res. 2013;73:3865–76. doi: 10.1158/0008-5472.CAN-12-3918. [DOI] [PubMed] [Google Scholar]
- 4.Neparidze N, Dhodapkar MV. Harnessing CD1d-restricted T cells toward antitumor immunity in humans. Ann N Y Acad Sci. 2009;1174:61–7. doi: 10.1111/j.1749-6632.2009.04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thapa P, Zhang G, Xia C, Gelbard A, Overwijk WW, Liu C, Hwu P, Chang DZ, Courtney A, Sastry JK, et al. Nanoparticle formulated alpha-galactosylceramide activates NKT cells without inducing anergy. Vaccine. 2009;27:3484–8. doi: 10.1016/j.vaccine.2009.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stirnemann K, Romero JF, Baldi L, Robert B, Cesson V, Besra GS, Zauderer M, Wurm F, Corradin G, Mach JP, et al. Sustained activation and tumor targeting of NKT cells using a CD1d-anti-HER2-scFv fusion protein induce antitumor effects in mice. J Clin Invest. 2008;118:994–1005. doi: 10.1172/JCI33249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, Rossjohn J, Perlmutter P, Cao J, Godfrey DI, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313–20. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 9.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]