Abstract
The presence of an immunosuppressive microenvironment can limit the full potential of adoptive T cell immunotherapy. However, specific blockade of the PD-1 immunosuppressive pathway can significantly enhance the function of gene-modified T cells expressing a chimeric antigen receptor (CAR) leading to enhanced tumor eradication.
Keywords: chimeric antigen receptor, adoptive immunotherapy, PD-1 blockade, cancer, immune response
Adoptive cell therapy (ACT) utilizing gene-modified T cells expressing chimeric antigen receptors (CAR) has emerged as a promising regimen for the treatment of a broad range of cancers including chronic lymphoid leukaemia and neuroblastoma with recent reports of long term remission in some patients.1,2 However, despite these encouraging outcomes, the existence of different immunosuppressive pathways can hinder the full potential of immunotherapy utilizing adoptively transferred T cells.3 The recent development of checkpoint inhibitors to specifically block these pathways has provided unique opportunities to effectively enhance T cell based immune therapies.3
Clinical trials targeting the programmed death-1 (PD-1) pathway using a human IgG4 PD-1 monoclonal antibody (mAb; BMS-936558) or human IgG4 anti-PD-L1 antibody, have reported strong clinical responses in patients against a range of solid cancers including advanced melanoma, non-small-cell lung, renal cell, as well as hematologic malignancies.4-6 Furthermore, a significant correlation between the level of PD-1 ligand (PD-L1) expression on tumor cells and objective responses was reported.4 Given these outstanding results, we sought to examine whether a combined immunotherapeutic approach involving blockade of the PD-1 pathway could enhance redirected T cell therapy.
Our hypothesis was that CAR T cell therapy in combination with PD-1 blockade may overcome PD-L1+ tumor immunosuppression, thereby leading to improved therapeutic efficacy. To test this, we first generated primary mouse T cells expressing an anti-Her-2 CAR containing the extracellular scFv-anti-Her-2 human monoclonal antibody region fused to the transmembrane and intracellular costimulatory domain CD28 and intracellular TCR-ζ domain, as previously described.7 Given that activation of T cells through TCR recognition of MHC/peptide has been shown to enhance PD-1 expression, we first assessed whether stimulation of T cells through the anti-Her-2 CAR could increase the level of expression of PD-1. In this experiment, we demonstrated a significant increase in PD-1 expression on CAR+ CD8+ T cells following antigen-specific stimulation with PD-L1+ Her-2+ tumor cells.8 Furthermore, we found that blockade of PD-1 with an anti-PD-1 mAb significantly enhanced the intracellular expression of the proliferation marker Ki-67, as well as IFNγ and granzyme B in CAR+ T cells.8 This suggested that blocking PD-1 immunosuppression could increase important functional parameters in CAR T cells following stimulation through the CAR and warranted testing this combined approach in an in vivo setting.
In adoptive transfer studies utilizing immune competent self-antigen Her-2 transgenic mice we had previously shown that adoptive transfer of anti-Her-2 CAR T cells alone could specifically mediate regression of 24JK-Her-2 experimental lung metastasis. This therapy however was less effective against established subcutaneous tumors.7 Hence, given the upregulation of PD-1 expression and increased functional capacity of CAR T cells following antigen stimulation in vitro, we investigated whether a combined approach of CAR T cells with PD-1 blockade could more effectively eradicate established tumors grown subcutaneously in Her-2 transgenic mice. In this experiment, we demonstrated significantly enhanced regression of 24JK-Her-2 sarcoma cells and survival of mice treated with anti-Her-2 CAR T cells in combination with an anti-PD-1 antibody.8 To demonstrate the broad utility of this combined approach, we also demonstrated that anti-Her-2 T cell and anti-PD-1 antibody treatment could significantly reduce the growth of established e0771-Her-2 breast carcinoma tumors injected orthotopically into Her-2 transgenic mice compared to either treatment alone.8
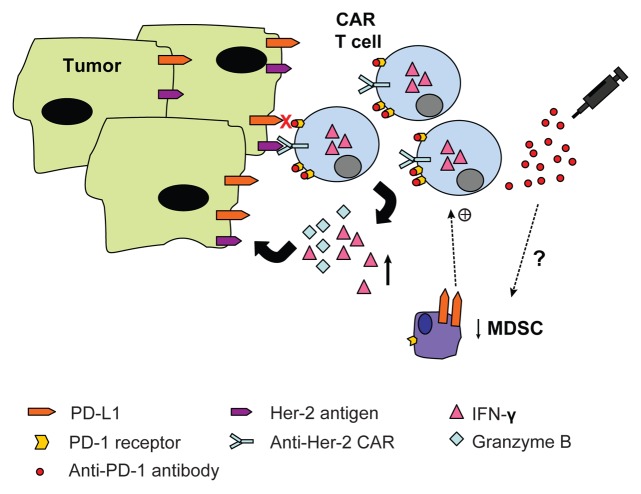
To gain better insight into the mechanism underlying this enhanced therapeutic response, we examined the frequency and function of CAR T cells within the tumor microenvironment following combined treatment. Although there was no observed increase in the percentage of CAR T cells following anti-PD-1 therapy, we did observe an increase in CAR T cell function as indicated by higher expression of intracellular IFNγ in these cells (Fig. 1).8 Given previous reports of anti-PD-1 treatment on decreasing the frequency of Treg and myeloid derived suppressor cells (MDSCs), we next investigated the possibility that anti-PD-1 treatment may have been enhancing CAR T cell anti-tumor responses by impacting on these immunosuppressive cell populations. Although there was no effect on percentage of Treg cells following combined treatment, we did observe a significant decrease in the percentage of Gr1+ CD11b+ MDSCs within the tumor microenvironment following PD-1 blockade (Fig. 1).8 The link between decreased MDSC numbers and enhanced anti-tumor effects following therapy requires further investigation however, it is likely that the reduction in MDSCs in our model was due to an indirect mechanism given the low level of expression of PD-1 on Gr1+ CD11b+ cells present at the tumor site.8
Figure 1. PD-1 blockade enhances CAR T cell therapy in vivo. Adoptive cell therapy using gene-modified T cells expressing a chimeric antigen receptor specific for the human Her-2 antigen in combination with anti-PD-1 antibody blockade results in increased functional capacity of CAR T cells following stimulation with PD-L1+ Her-2+ tumor targets. A concomitant decrease in percentage of myeloid derived suppressor cells (MDSC’s) within the tumor microenvironment was observed that may have contributed to enhancing CAR T cell function (+).
Finally, we assessed the safety of this combined therapy given previous reports of toxicity in some CAR T cell9 and anti-PD-1 trials.4 We utilized the Her-2 transgenic mouse model which constitutively expresses the human Her-2 antigen in the brain (cerebellum) and breast tissue, to examine potential pathology to normal tissue. Using immunohistochemical analysis, we compared both brain and breast tissue from mice that received adoptive transfer of CAR T cells alone or in combination with anti-PD-1 antibody. Our results revealed no pathology to Her-2+ brain or mammary tissues in any of the treatment groups.8 This safety data is important for moving this combined immunotherapeutic approach towards a Phase I clinical trial.
Overall, our work demonstrates for the first time that blockade of PD-1 immunosuppression can significantly enhance the therapeutic efficacy of CAR T cell therapy against established solid cancers. The use of a self-antigen model in our preclinical studies indicated that the combined approach was both effective and safe. Our findings open up the distinct possibility that blockade of other inhibitory receptors such as T cell membrane protein-3 (TIM-3) may further enhance CAR T cell therapy. Taken together, our data has significant implications for potentially improving therapeutic outcomes of CAR T cell therapy in cancer patients that have not effectively responded to first line treatments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: John LB, Kershaw MH, Darcy PK. PD-1 blockade boosts CAR-expressing T cell-based immunotherapy. OncoImmunology 2013; 2:e26286; 10.4161/onci.26286
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26286
References
- 1.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LXJ, Westwood JA, Moeller M, Duong CPM, Wei W-Z, Malaterre J, Trapani JA, Neeson P, Smyth MJ, Kershaw MH, et al. Tumor ablation by gene-modified T cells in the absence of autoimmunity. Cancer Res. 2010;70:9591–8. doi: 10.1158/0008-5472.CAN-10-2884. [DOI] [PubMed] [Google Scholar]
- 8.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Liu L, Wang Z. Improving the efficacy and safety of engineered T cell therapy for cancer. Cancer Lett. 2013;328:191–7. doi: 10.1016/j.canlet.2012.09.015. [DOI] [PubMed] [Google Scholar]