Abstract
The inspection of the mechanisms through which autophagy modulates immunogenic cell death revealed that the autophagic response of cancer cells to reactive oxygen species-dependent endoplasmic reticulum stress suppresses the exposure of calreticulin on the cell surface, the phenotypic maturation of dendritic cells (DCs) as well as their ability to release interleukin-6 and to support the proliferative expansion of (interferon γ-producing) CD4+ and CD8+ T lymphocytes. These findings unveil an unprecedented role for therapy-induced autophagy in suppressing key mechanisms that underlie anticancer immune responses as elicited by immunogenic cell death.
Keywords: chaperone-mediated autophagy, calreticulin, damage-associated molecular patterns, dendritic cells, immunogenic cell death, photodynamic therapy, reactive oxygen species, T lymphocytes
Immune responses against established neoplasms are often dysfunctional due the fact that cancer cells are poorly immunogenic and highly immunoevasive.1 Various studies have demonstrated that the elicitation of anticancer immune responses along with the death of malignant cells can provide long-lasting therapeutic effects.1 Unfortunately, most cytotoxic therapies are incapable of reinstating the immunogenicity of cancer cells.2 However, it has recently been observed that some anticancer agents including assorted chemotherapeutics, hypericin-based photodynamic therapy (Hyp-PDT) and radiotherapy3 can induce an immunostimulatory form of cellular demise known as immunogenic cell death (ICD),1,4 which favors the (re)activation of anticancer immunity.1,2,4 At least in part, ICD has immunogenic effects owing to the emission by dying cancer cells of immunostimulatory molecules called damage-associated molecular patterns (DAMPs).2,3 DAMPs that are crucial for ICD include: (1) the exposure of calreticulin (CRT) on the cell surface, which provides an “eat me” signal; (2) the secretion of ATP, which stimulates the inflammasome-dependent production of interleukin (IL-1β), in turn promoting the functional polarization of T cells; (3) the release of high mobility group box 1 (HMGB1) or other Toll-like receptor (TLR) agonists like heat-shock protein 70 (HSP70), which promotes proper antigen processing within antigen-presenting cells.2-4
Notably, macroautophagy (hereafter referred to as autophagy) has been found to act as a “switchable mechanism” during oncogenesis and tumor progression, exerting tumor-suppressive or tumor-promoting functions in a contextual manner.5 Since most anticancer therapies induce an autophagic response,1,5,6 it has become crucial to analyze how therapy-induced autophagy modulates the cancer cell-immune system interface.4 We have recently shown that the localized generation of reactive oxygen species (ROS) at the endoplasmic reticulum (ER), as triggered by the activation of the reticulotropic photosensitive drug hypericin with light, results in the efficient induction of ICD. In this setting, ICD relies on cell death coupled to the activation of danger signaling by photo-oxidative ER (phox-ER) stress, the therapeutic principle behind Hyp-PDT (Fig. 1A).2,7,8 However, since phox-ER stress also induces a wide cytoprotective autophagic response, encompassing macroautophagy and chaperone-mediated autophagy (CMA, a more selective form of autophagy for the clearance of damaged/oxidized soluble proteins)9,10 (Fig. 1B); it became imperative to analyze the role of autophagy in this ICD paradigm.
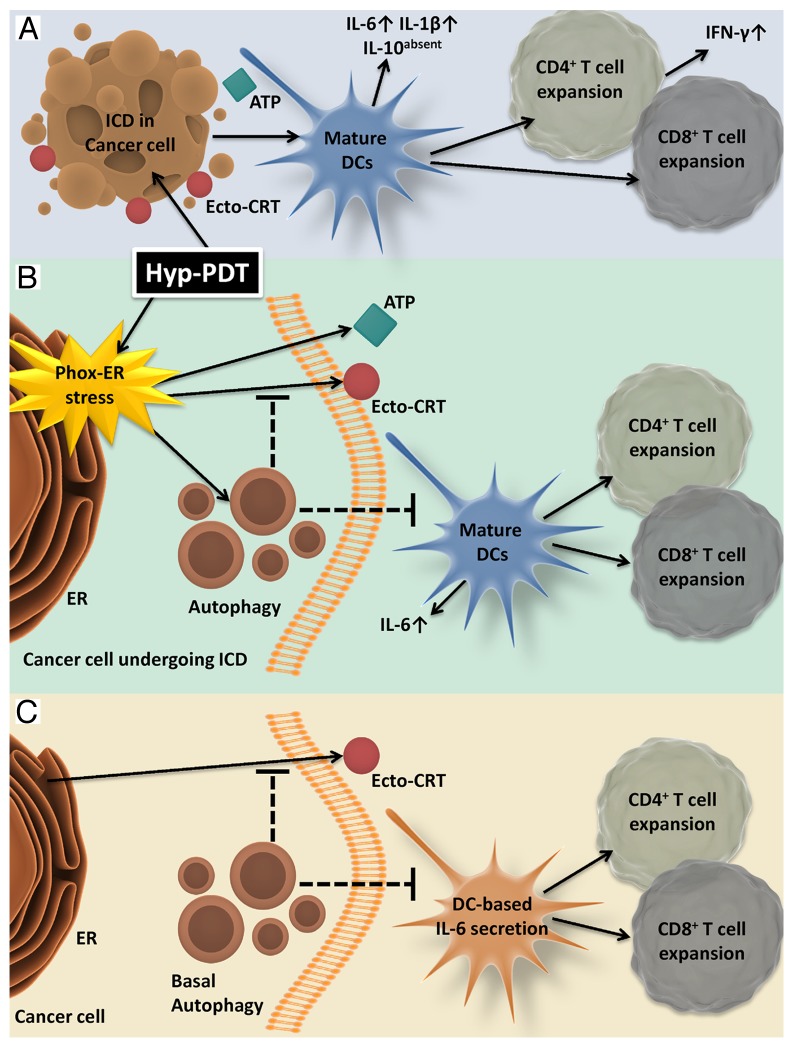
Figure 1. Autophagy induced in the course of hypericin-based photodynamic therapy dampens the immunogenicity of cell death and hence inhibits the elicitation of anticancer immune responses. (A) Photo-oxidative endoplasmic reticulum (phox-ER) stress induced by hypericin-based photodynamic therapy (Hyp-PDT) stimulates the efficient pre-apoptotic trafficking of damage-associated molecular patterns (DAMPs) including calreticulin (CRT) and ATP toward the extracellular environment. Hyp-PDT-induced ICD potently induces the phenotypic maturation of dendritic cells (DCs) as well as their functional activation, characterized by the production of immunostimulatory cytokines such as interleukin (IL)-6 and IL-1β in absence of immunosuppressive IL-10. In turn, activated and mature DCs induce potent cellular immune responses featuring the proliferative expansion of CD4+ and CD8+ T lymphocytes that secrete interferon γ (IFNγ) production. (B) Hyp-PDT-stress induced autophagy dampens ER stress, the accumulation of oxidized proteins and the exposure of CRT on the cell surface (but not ATP secretion), hence inhibiting the maturation of DCs as well as their ability to secrete IL-6 and to sustain the proliferation of (IFNγ-producing) CD4+/CD8+ T lymphocytes. (C) Baseline levels of autophagy in untreated/healthy cancer cells also suppress CRT exposure, IL-6 production by DCs and the proliferation of CD4+ and CD8+ T lymphocytes.
We found that cancer cells subjected to the stable knockdown of ATG5, a maneuver that inhibits autophagy, manifest not only an increased proteotoxic response (i.e., the accumulation of oxidatively damaged proteins and the elicitation of ER stress) to Hyp-PDT, but also a considerable increase in surface-exposed CRT (ecto-CRT), reflecting a robust correlation between the levels of ecto-CRT, ER stress and proteotoxicity.6 Interestingly, the secretion of ATP by cancer cells exposed to Hyp-PDT was not affected by ATG5KD.6 Furthermore, the knockdown of ATG5 improved the ability of cancer cells undergoing phox-ER stress-elicited ICD to trigger the phenotypic maturation of DCs as well as the DC-mediated secretion of IL-6 (without affecting IL-10/IL-1β production).6 This inhibition of autophagy also led to a significantly increased capacity of DCs to support the proliferative expansion of interferon γ (IFNγ)-producing CD4+ and CD8+ T lymphocytes.6 Altogether, these results demonstrate for the first time that the activation of autophagy by phox-ER stress dampens the immunogenicity of ICD (as mediated by ecto-CRT) and inhibits key processes that are required for the elicitation of anticancer immune responses (as mediated by DCs as well as CD4+ and CD8+ T lymphocytes), at least in vitro (Fig. 1B).
These results are unprecedented because the activation of autophagy in the course of ICD induced by chemotherapeutics such as mitoxantrone and oxaliplatin was found to facilitate ATP secretion (without affecting ecto-CRT levels), the intratumoral accumulation of activated DCs and T cells as well as the T cell-mediated production of IFNγ.4 As the content of autophagosomes may differ depending on the subcellular localization and nature of the stress,5 we postulate that these contradictory results may be explained by differences in biochemical features of the autophagic cargo, which presumably consists of a considerable amount of oxidized cytoplasmic structures in cells treated with Hyp-PDT.10 Interestingly, we observed that cells lacking lysosomal-associated membrane protein 2A (LAMP2A), a lysosomal CMA- specific receptor, fail to expose CRT upon treatment with chemotherapy or Hyp-PDT, while their capacity to secrete ATP is unaffected.9 Such a strong dysfunction could not be rescued by the knockdown of ATG5, even though the baseline levels of autophagy in LAMP2A-deficient cells are increased as a compensatory mechanism.9 Considering that absence of the integral lysosomal protein LAMP1 was shown to abolish autophagy-dependent ATP secretion in response to chemotherapy,4 these results raise the possibility that besides their degradative functions, lysosomes coordinate trafficking and delivery pathways for the emission of specific DAMPs, a very interesting conjecture that deserves further attention.
Of note, the knockdown of ATG5 in otherwise untreated human cancer cells increased their own levels of ecto-CRT, the secretion of IL-6 by interacting DCs and the ability of DCs to support the expansion of IFNγ-producing CD4+ and CD8+ T lymphocytes.6 Thus, our observations suggest that, at least in the setting of human melanoma, cancer cell-intrinsic autophagy might assist in evasion from immunosurveillance by suppressing basal ecto-CRT levels and restraining the activation of cellular immunity (Fig. 1C).
Two major corollary questions arise from these studies. First, is the autophagy-mediated suppression of immunogenicity specific for ICD as triggered by Hyp-PDT or is this a general process? Second, could the upregulation of autophagic response5 support a ‘tolerogenic’ cancer phenotype? Further studies are required to address these issues and clarify the impact of autophagy on the regulation of both cell-autonomous (e.g., redox status, ER stress, proteostasis, trafficking mechanisms) and non-autonomous mechanisms (e.g., secretion of immunological modulators) for the avoidance of antitumor immunity.
In conclusion, we observed that Hyp-PDT-induced autophagy dampens key processes that underlie ICD and the elicitation of anticancer immune responses (Fig. 1B), thus assisting cancer cells in the evasion from immunosurveillance (Fig. 1C). Thus, depending on the cancer model and the type of ICD inducer, autophagy might assist or prevent anticancer immunity. In the future, it will be interesting to characterize which immunosuppressive/tolerogenic factors responsible for the immunoevasive behavior of cancer cells are regulated by autophagy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Garg AD, Dudek AM, Agostinis P. Autophagy-dependent suppression of cancer immunogenicity and effector mechanisms of innate and adaptive immunity. OncoImmunology 2013; 2:e26260; 10.4161/onci.26260
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26260
References
- 1.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 2.Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.48. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013;24:319–33. doi: 10.1016/j.cytogfr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzzi L, Perfettini JL, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.75. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathew R, White E. Autophagy, stress, and cancer metabolism: what doesn’t kill you makes you stronger. Cold Spring Harb Symp Quant Biol. 2011;76:389–96. doi: 10.1101/sqb.2012.76.011015. [DOI] [PubMed] [Google Scholar]
- 6.Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, Mathieu C, Agostinis P. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013 doi: 10.4161/auto.25399. [DOI] [PubMed] [Google Scholar]
- 7.Garg AD, Krysko DV, Vandenabeele P, Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother. 2012;61:215–21. doi: 10.1007/s00262-011-1184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–79. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg AD, Dudek AM, Agostinis P. Calreticulin surface exposure is abrogated in cells lacking, chaperone-mediated autophagy-essential gene, LAMP2A. Cell Death Dis. 2013;4:e826. doi: 10.1038/cddis.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubio N, Coupienne I, Di Valentin E, Heirman I, Grooten J, Piette J, Agostinis P. Spatiotemporal autophagic degradation of oxidatively damaged organelles after photodynamic stress is amplified by mitochondrial reactive oxygen species. Autophagy. 2012;8:1312–24. doi: 10.4161/auto.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]