Introduction
Bilateral inferior petrosal sinus sampling (BIPSS) is gold standard test for anatomical localization for Cushing's disease.1 Initial reports on selective petrosal sinus sampling to differentiate pituitary and ectopic source of adrenocorticotropic hormone (ACTH) were performed by Corrigan published in 1977.2 Earlier, ratio of unilateral IPSS with peripheral venous ACTH levels was assessed but later on it was realized that pituitary venous drainage can be asymmetric either because of adenoma location or anatomical variation. Thus simultaneous BIPSS came into practice.3 In a meta-analysis of 21 studies, the overall sensitivity and specificity of BIPSS were found to be 96% and 100%4 respectively which can be improved after stimulation with CRH or desmopressin. Here we report a case of Cushing's disease with negative MRI brain for adenoma where BIPSS with stimulation with vasopressin confirmed the diagnosis and provided lateralization. She underwent successful surgical adenomectomy. Histopathology revealed pituitary adenoma and post operative cortisol level was consistent with remission.
Case report
A 19 years old girl presented with complaints of weight gain, hypertrichosis, menstrual irregularities of 2 years, and hypertension of 4 months duration. She also gave history of easy bruisability, broad violaceous striae over abdomen and thighs, and recurrent vaginal candidiasis. On examination her height was 154 cm with weight of 77 kg and BMI of 32 kg/m2. Her waist hip ratio was 0.95. She was normotensive on anti hypertensive medications. Examination revealed thinning of scalp hairs, mooning of face, facial plethora, hypertrichosis over face, increased supraclavicular and dorsocervical fat pad thickness, violaceous abdominal striae, thinning of skin over dorsum of hands and proximal muscle weakness. There was no neurological deficit including visual fields.
Investigation showed – hemoglobin – 13.6 g/dl, total leucocyte count – 8900/cumm, serum creatinine – 1.0 mg/dl, serum bilirubin – 0.4 mg/dl, serum calcium – 9.4 mg/dl, serum phosphorus – 3.8 mg/dl, and serum alkaline phosphatase – 120 U/l. Oral glucose tolerance test detected impaired glucose tolerance (fasting plasma glucose 98 mg/dl and post glucose load 145 mg/dl). Her lipid profile (serum triglyceride – 119; cholesterol – 170, LDL – 109 and HDL – 37 mg/dl) and thyroid profile (Free T3 – 2.99 pg/ml; Free T4 – 1.43 ng/ml; TSH – 4.76 μIU/ml) were normal. Her bone mineral density estimation by DXA revealed low bone mineral density (Z score at lumbar spine was −2.3 and femoral neck −1.2). Electrocardiogram and chest X-ray were within normal limits.
Her basal ACTH and cortisol levels were elevated (38.7 pg/ml and 33.5 μg/dl respectively). Diurnal changes of cortisol were not observed (8 a.m. – 21.3 μg/dl; 4 p.m. – 23 μg/dl). Serum cortisol was non suppressible with overnight (serum cortisol – 25.26 μg/dl) and low dose (serum cortisol – 12.66 μg/dl) dexamethasone suppression test. However; high dose dexamethasone decreased cortisol value by 73% (33.5 μg/dl to 7.7 μg/dl).
Imaging studies with MRI Brain did not reveal any pituitary adenoma (Fig. 2). In view of non localization of source of ACTH and reluctance by neurosurgeons to operate without localization, BIPPS was planned. BIPSS was performed with vasopressin due to non availability of CRH and desmopressin for the first time in any service hospital to the best of our knowledge.
Fig. 2.
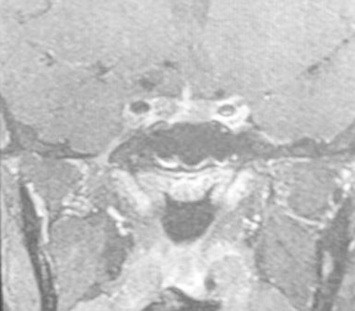
MRI Pituitary showing normal pituitary.
BIPSS
Catheterization of both inferior petrosal sinuses was performed through a percutaneous bilateral femoral vein approach. After a catheter was advanced into a petrosal sinus, a small amount of contrast material was injected to verify the location of the catheter tip. Also contrast was seen going from one side and returning from other side through cavernous sinus (Fig. 1), confirming correct catheterization. Blood was slowly withdrawn from both catheters simultaneously and from ipsilateral peripheral veins for ACTH measurement at −5 and −1 min. Vasopressin (1.0 Unit diluted in 10 ml saline over 5 min) was then infused into a peripheral vein, and samples were simultaneously obtained from both inferior petrosal sinuses and peripheral veins 5 and 10 min after the administration of vasopressin in cold test tube.
Fig. 1.
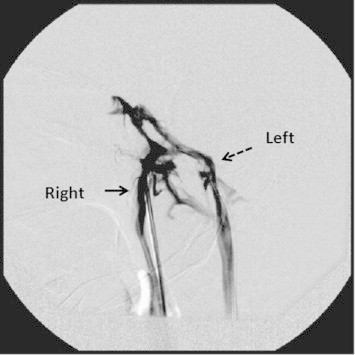
Bilateral inferior petrosal sinus.
ACTH assay
ACTH is a fragile peptide, which is rapidly degraded by peptidase present in blood at room temperature. To circumvent spurious result, we established the assay in our laboratory at the same time of BIPSS. Samples were transported in cold environment, and serum was separated under cold centrifugation. ACTH assay was performed using immunoradiometric assay kit (Immunotech, Beckman Coulter Company) using Stratec SR300 automated radioimmunoassay system. The intra-assay and inter-assay coefficient of variation were below or equal to 9.1% and 9.6% respectively. ACTH values were used to calculate the ratio of ACTH between the right or left inferior petrosal sinus and the concentration in the peripheral blood (IPS:P ratio) and the maximal ratio (right or left) was calculated. Sampling giving the highest value of ACTH (5 or 10 min after the injection of vasopressin) was used to determine the ratio. ACTH values and gradients are depicted in Table 1. Central to peripheral ACTH ratio on right side was 28/1 and 32/1 in basal and post vasopressin respectively. The interpetrosal sinus gradient was also significant (Right IPS/left IPS = 13) suggesting pituitary microadenoma on right side of adenohypophysis. Patients pulse rate and blood pressure varied little with injection vasopressin. There were no other perioperative or post procedure complications.
Table 1.
Serum ACTH Levels during BIPSS.
| Time | −5 min | −1 min | 5 min after vasopressin | 10 min after vasopressin |
|---|---|---|---|---|
| Right IPSa | 519.4 | 851.4 | 1705 | 648 |
| Left IPS | 93.96 | 132.7 | 131 | 80 |
| Right femoral vein | 18.48 | 31.11 | 53 | 26 |
| Left femoral vein | 23.21 | 33.26 | 36 | 33 |
| Central/Peripheral ACTH ratio | Right = 28/1 Left = 4/1 |
Right = 27/1 Left = 4/1 |
Right = 32/1 Left = 3.6/1 |
Right = 25/1 Left = 2.4/1 |
| Right IPS/Left IPS | 5.6/1 | 6.5/1 | 13/1 | 8/1 |
IPS – Inferior petrosal sinus.
The patient was diagnosed as having Cushing's disease caused by an ACTH-secreting pituitary adenoma, which was extirpated by selective adenomectomy through trans-sphenoidal surgery. The tumor was totally removed and was soft and light yellowish-white (typical features of pituitary adenomas). Histopathology confirmed pituitary adenoma with strongly positive immunohistochemical staining with ACTH monoclonal antibodies (Fig. 3). Sixth post operative day basal cortisol levels were 0.33 μg/dl; suggestive of possible remission of disease. She was discharged on replacement doses of hydrocortisone with advice on stress dosing and she is planned for gradual tapering of hydrocortisone doses.
Fig. 3.
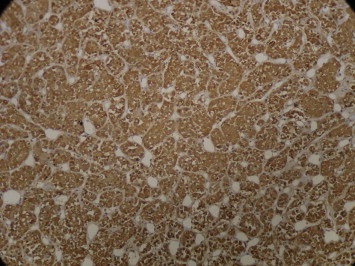
Immunohistochemical staining 20×: tumor cells showing diffuse strong positivity for ACTH monoclonal antibodies.
Discussion
Cushing's disease is clinically a spot diagnosis however its anatomical localization to pituitary as source of excess ACTH, specially differentiating from ectopic sources of ACTH can be quite difficult.5 It is associated with marked morbidity and mortality thus its definitive treatment is mandatory which requires localization of ACTH source to pituitary.6 Cushing's disease is caused by microadenomas (<1 cm) in more than 90% of cases. In only 36–78% of cases MRI is able to localize the adenoma in pituitary because of very small sized adenoma or diffuse corticotroph hyperplasia.7 However, dynamic studies have improved sensitivity but, still in many cases MRI fail to localize the adenoma as happened in our case.8 About 10% of general population may be harboring incidentaloma in pituitary, which may result in false positivity and failed surgery.9
Here is the role of BIPSS, which depicts central to peripheral gradient of ACTH in basal conditions and which becomes even more pronounced after corticotrophs are stimulated for production of ACTH by exogenous agent. It can also serve to lateralize the adenoma in pituitary which can be helpful intra-operatively. BIPSS is considered as gold standard in differentiating pituitary dependant Cushing's syndrome from ectopic Cushing's syndrome.1 Basal IPS/PV ratio of ACTH more than 2, and post stimulation IPS/PV if more than 3 is considered to be diagnostic of Cushing's disease. Also lateralization of pituitary adenoma can be predicted if interpetrosal ratio of ACTH is more than 1.5. However, it can be false negative (BIPSS predicts non-pituitary Cushing's in the presence of a corticotroph adenoma) when there is aberrant pituitary venous drainage or incorrect BIPSS technique (inability to cannulate each inferior petrosal sinus or dislodgement during the procedure).6 Hence, it is important to ascertain catheter position as done in our case. BIPSS has over all been reported to have sensitivity and specificity of 96% and 100% respectively.
BIPPS has been performed in any Indian army hospital for the first time. In India there is scarce data about BIPSS use in Cushing's disease; firstly, because of invasive procedure of BIPSS and associated risk factors, secondly, its high cost, thirdly, intervention radiology in India is in its infancy and finally, non availability of injection CRH, desmopressin in India. Corticotrophs are stimulated to produce ACTH by corticotrophin releasing hormone (CRH). Following CRH, maximal data is available for use of injection desmopressin in combination with CRH. Desmopressin has been used alone for stimulation of corticotrophs during BIPSS with satisfactory results.10 Neither CRH nor desmopressin is available in India. Hence, we innovatively tried vasopressin for the corticotroph stimulation. Vasopressin acts on V1 receptors on vascular smooth cells exerting weak vasopressor effects; on V2 receptors it has its anti diuretic action. V3 receptors are found on corticotrophic cells in the anterior pituitary where classically it has been shown to have weak ACTH secretogogues effect in isolation, while acting synergistically with CRH it causes significant secretion of ACTH.11 In our case we could demonstrate significant increment in ACTH values after injection Vasopressin and lateralization of adenoma to the right side of adenohypophysis.
Treatment of choice for Cushing's disease is surgical adenomectomy by trans-nasal trans-sphenoidal route. Results of surgery are dependent on surgeon's expertise and cure rates of approximately 80–90% for microadenomas and 50% for macroadenomas.12 Post operatively patient should be assessed for remission of disease by evaluation of serum cortisol within first post operative week. If basal (09:00 a.m.) cortisol levels are less than 2 μg/dl then there is 10% chances of remission after 10 years of surgery.13 In our case serum cortisol on sixth day was <1 μg/dl, which was consistent with remission and low probably of recurrence. However, patient is kept under follow-up on suboptimal doses of hydrocortisone to help recovery of hypothalamo-pituitary adrenal axis.
Here, we report a case of Cushing's disease where we performed bilateral IPSS with vasopressin stimulation with satisfying results for the first time in Indian army. We would like to recommend that in view of non availability of CRH or desmopressin in India vasopressin may be used instead during further studies for stimulation during BIPSS.
Conflicts of interest
All authors have none to declare.
References
- 1.Lad S.P., Patil C.G., Laws E.R., Jr., Katznelson L. The role of inferior petrosal sinus sampling in the diagnostic localization of Cushing's disease. Neurosurg Focus. 2007;23:E2. doi: 10.3171/foc.2007.23.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Corrigan D., Schaff M., Whaley R., Czerwinski C., Earll J. Selective venous sampling to differentiate ectopic ACTH secretion from pituitary Cushing's syndrome. NEJM. 1977;196:861–862. doi: 10.1056/NEJM197704142961508. [DOI] [PubMed] [Google Scholar]
- 3.Doppman J., Oldfield E., Krudy A. Petrosal sinus sampling for Cushing syndrome: anatomical and technical considerations. Work in progress. Radiology. 1984;150:99–103. doi: 10.1148/radiology.150.1.6316418. [DOI] [PubMed] [Google Scholar]
- 4.Newell-Price J., Trainer P., Besser M., Grossman A. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev. 1998;19:647–672. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 5.Ezzat S., Asa S., Couldwell W. The prevalence of pituitary adenomas. Cancer. 2004;101:613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 6.Utz A., Biller M.K. The role of bilateral inferior petrosal sinus sampling in the diagnosis of Cushing's syndrome. Arq Bras Endocrinol Metab. 2007;51(8) doi: 10.1590/s0004-27302007000800019. [DOI] [PubMed] [Google Scholar]
- 7.Testa R.M., Albiger N., Occhi G. The usefulness of combined biochemical tests in the diagnosis of Cushing's disease with negative pituitary magnetic resonance imaging. Eur J Endocrinol. 2007;156:241–248. doi: 10.1530/eje.1.02332. [DOI] [PubMed] [Google Scholar]
- 8.Kaskarelis S.O., Tsatalou E.G., Benakis S.V. Bilateral inferior petrosal sinuses sampling in the routine investigation of Cushing's syndrome: a comparison with MRI. AJR Am J Roentgenol. 2006;187:562–570. doi: 10.2214/ajr.05.0557. [DOI] [PubMed] [Google Scholar]
- 9.Molitch M.E., Russell E.J. The pituitary “incidentaloma”. Ann Intern Med. 1990;112:925–931. doi: 10.7326/0003-4819-112-12-925. [DOI] [PubMed] [Google Scholar]
- 10.Castinetti F., Morange I., Dufour H. Desmopressin test during petrosal sinus sampling: a valuable tool to discriminate pituitary or ectopic ACTH-dependent Cushing's syndrome. Eur J Endocrinol. 2007;157:271–277. doi: 10.1530/EJE-07-0215. [DOI] [PubMed] [Google Scholar]
- 11.Carmody D., Hannon M.J., Thompson C. Vasopressin, diabetes insipid and the syndrome of inappropriate ADH secretion. In: Jameson J.L., DeGroot L.J., editors. Endocrinology Adult and Pediatrics. 6th ed. Elsevier Saunders; Philadelphia: 2010. pp. 386–399. [Google Scholar]
- 12.Utz A.L., Swearingen B., Biller B.M. Pituitary surgery and postoperative management in Cushing's disease. Endocrinol Metab Clin North Am. 2005;34:459–478. doi: 10.1016/j.ecl.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay J.R., Oldfield E.H., Stratakis L.A., Niemann L.K. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing's disease after transsphenoidal surgery. J Clin Endocrinol Metab. 2011;96:2057–2064. doi: 10.1210/jc.2011-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]


