Abstract
Background
Autophagy has been reported to be essential for pre-implantation development and embryo survival. However, its role in placental development and regulation of autophagy during pregnancy remain unclear. The aims of this study were to (1) study autophagy by characterizing changes in levels of beclin-1, DRAM, and LC3B in human placenta throughout gestation; (2) determine whether autophagy is involved in regulation of trophoblast invasion in JEG-3 cells (a choriocarcinoma cell line); (3) examine the effects of reduced oxygen and glucose on the autophagic changes; and (4) investigate the effect of reoxygenation and supplementation of glucose after oxygen-glucose deprivation (OGD) on the autophagic changes in primary cytotrophoblasts obtained from normal term pregnancy.
Methodology/Principal Findings
An analysis of 40 placental samples representing different gestational stages showed (1) no significant differences in beclin-1, DRAM, and LC3B-II levels in placentas between early and mid-gestation, and late gestation with vaginal delivery; (2) placentas from late gestation with cesarean section had lower levels of LC3B-II compared to early and mid-gestation, and late gestation with vaginal delivery; levels of DRAM were also lower compared to placentas from early and mid-gestation; and (3) using explant cultures, villous tissues from early and late gestation had similar rates of autophagic flux under physiological oxygen concentrations. Knockdown of BECN1, DRAM, and LC3B had no effects on viability and invasion activity of JEG-3 cells. On the other hand, OGD caused a significant increase in the levels of LC3B-II in primary cytotrophoblasts, while re-supplementation of oxygen and glucose reduced these changes. Furthermore, there were differential changes in levels of beclin-1, DRAM, and LC3B-II in response to changes in oxygen and glucose levels.
Conclusions/Significance
Our results indicate that autophagy is involved in development of the human placenta and that changes in oxygen and glucose levels participate in regulation of autophagic changes in cytotrophoblast cells.
Introduction
Autophagy is a highly regulated process involving invagination and degradation of cytoplasmic components such as damaged organelles, protein aggregates, and invading organisms through the lysosomal pathway [1]. During autophagy, proteins and organelles are sequestered into double-membrane vesicles called autophagosomes. Autophagosomes ultimately fuse with lysosomes to generate single-membrane autophagolysosomes and the sequestered material is degraded and metabolized by the cell for macromolecular synthesis and energy generation. These autophagic functions are important for cellular homeostasis and bioenergic management, and promote cell survival in response to environmental stresses such as starvation and hypoxia.
In mammals, autophagy has been reported to be essential for pre-implantation development and in regulation of embryo survival [2]. Autophagy-related proteins such as beclin-1, LC3B, and DRAM have been shown to be present in the human placenta [3-5]. Beclin-1 is a part of an early complex that promotes synthesis and growth of pre-autophagosomal membranes [6]. LC3B is synthesized as proLC3B and converted to LC3B-I by autophagy-related proteases. Upon induction of autophagy, LC3B-I is further processed into LC3B-II and integrated into membranes of autophagosomes [6]. DRAM is a lysosomal protein that regulates autophagy in a p53-dependent manner [7]. Compared to normal pregnant women, increased autophagy has been noted in placentas from women with pregnancies complicated by fetal growth restriction (FGR) or severe preeclampsia [3-5]. In association with apoptosis, autophagy has been found to be involved in the process of membrane rupture of human amnion in term gestation [8]. Furthermore, hypoxia induces apoptotic and autophagic changes in primary human cytotrophoblasts [4,9], and there are differential changes in autophagy and apoptosis of trophoblasts between constant oxygen conditions and hypoxia-reoxygenation [10]. Notably, cytotrophoblasts transfected with BECN1, LC3B, or DRAM siRNA showed increased apoptosis under hypoxia compared to controls, suggesting a protective role for autophagy against hypoxia-induced trophoblast apoptosis [4]. Nevertheless, autophagic changes in human placenta throughout gestation and the mechanisms underlying the regulation of autophagy in placental development remain unclear.
Given the fact that autophagy plays an important role in early embryo development and in pregnancy complications, the specific objectives of this study are as follows: (1) to study the extent of autophagy by characterizing changes in the levels of beclin-1, LC3B, and DRAM in the human placenta throughout gestation; (2) to ascertain whether autophagy is involved in regulation of trophoblast invasion activity using a choriocarcinoma cell line, JEG-3 cells, as a model; (3) to examine the effect of reduced oxygen and glucose on autophagic changes; and (4) to investigate the effect of reoxygenation and supplementation of glucose on autophagic changes after oxygen-glucose deprivation (OGD) in primary cytotrophoblasts obtained from normal term pregnancy.
Materials and Methods
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taiwan (No. 98-3987B). All placental samples were collected after the subjects had provided written informed consent.
Collection of placental tissues
Villous samples were obtained from the following sources or scenarios: elective termination of gestations ranging from 7 to 24 weeks with documented embryonic or fetal cardiac activity by ultrasound (n = 16); pregnancies complicated by cervical incompetence (n = 2); spontaneous preterm deliveries ranging from 24 to 28 weeks in the absence of signs, symptoms, and histological evidence of chorioamnionitis (n = 2); elective repeat cesarean section (CS) prior to the onset of labor (38 to 39 weeks, n = 10); and uneventful vaginal deliveries (VD) (38 to 40 weeks, n = 10). In the current study, villous samples were grouped according to gestational age and delivery mode as follows: (1) early gestation (7–12 weeks, n = 10), (2) mid-gestation (13–28 weeks, n = 10), (3) late gestation with CS (38–39 weeks, n = 10), and (4) late gestation with VD (38–40 weeks, n = 10). Villous samples from early gestation were submitted for chromosomal study to confirm normal karyotypes. All fetuses delivered were examined by obstetricians for any gross malformations.
For mid- and late gestation, villous tissues were randomly sampled from five distinct sites from the maternal side after the placenta was delivered. Each site was midway between the cord insertion and the periphery of the placenta and midway between the chorionic and basal plates. The villous samples were quickly washed in ice-cold phosphate-buffered saline to clear the maternal blood, frozen in liquid nitrogen, and stored at -70°C for further processing. All villous samples were collected and processed within 10 min after delivery.
Isolation and culture of cytotrophoblast cells from term placentas
Isolation and culture of cytotrophoblast cells from normal-term placentas were performed as previously described [11]. The purified cells were then plated at a minimum of 2 × 105 cells/cm2 in 6-well plates and cultured in a humidified atmosphere with 5% CO2 and balanced air (standard conditions, designated as 18% O2 with glucose in this study). After an overnight rest, the cells were rinsed twice with pre-warmed culture medium (RPMI 1640 containing D-glucose at 2 mg/mL, 5% fetal bovine serum, antibiotics, and antimycotics; all from Invitrogen, Life Technologies, Grand Island, NY, USA) to remove non-attached cells, and the medium was changed every 24 to 48 h.
To study the effect of reduced oxygen and glucose concentrations on the changes in autophagy-related proteins, cytotrophoblasts were cultured in a medium with or without glucose in 2% or 18% O2 with 5% CO2/balanced N2. After 48 h of incubation, the cell lysates were collected and stored at -70°C for further processing.
To study the effect of supplementation of oxygen and glucose on the autophagic changes after OGD, cytotrophoblasts were cultured in a medium without glucose in 2% O2/5% CO2/balanced N2 conditions for 48 h and then subjected to the following 4 conditions: (1) 2% O2 without glucose; (2) 2% O2 with glucose; (3) 18% O2 without glucose; and (4) 18% O2 with glucose. After 48 h, the cell lysates were collected and stored at -70°C for further processing.
Villous explant culture
To compare the autophagic flux between villous tissues of early and late gestation, explants prepared from villous tissues of early gestation (7–10 weeks, n = 5) and from placentas of term pregnancy after elective CS (38–39 weeks, n = 5) were cultured under 2% and 8% O2 with 5% CO2 and balanced N2, respectively. After soaking overnight, the medium was changed, and the explants were cultured in duplicate individually with or without 200 nM of bafilomycin A1 (Sigma-Aldrich, St. Louis, MO, USA) for 48 h.
Transfection of small interfering RNAs (siRNAs) for BECN1, LC3B, and DRAM in JEG-3 cells
Specific siRNAs for human BECN1 (sc-29797), LC3B (MAP LC3β, sc-43390), and DRAM (sc-96209), as well as control siRNAs (sc-44230 and sc-44231), were purchased from Santa Cruz Biotechnology, Dallas, TX, USA. Transfection of siRNA was performed using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturers’ instructions. siRNA transfection was performed under serum deprivation conditions, as previously detailed [4]. The siRNA/transfection reagent mixture was overlaid on the cells and incubated at 37°C in a humidified CO2 incubator. After 6 h, the overlaid siRNA/transfection reagent mixture was removed and replaced with fresh culture medium and incubated under standard conditions. Control siRNAs, each containing a scrambled sequence that will not lead to the specific degradation of any known cellular mRNA, were used as negative controls.
Cell viability assay
Cell viability was determined by assessing the degree of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromides; Sigma) reduction. At a certain time in an individual experiment, cells were incubated at 37°C in 1 mg/mL MTT solution for 2 h. After removal of the MTT solution, dimethyl sulfoxide was added, and the absorbance at a wavelength of 490 nm was recorded with a microplate reader. Cell viability was expressed as a percentage of the control culture. All experiments were performed in triplicate.
Transwell insert invasion assay
Transwell insert invasion assays were conducted in 24-well fitted inserts with membranes (8 µm pore size; BioCoat Matrigel Invasion Chamber, BD Biosciences, Bedford, MA, USA). Briefly, JEG-3 cells were trypsinized after transfection, seeded into transwell inserts pre-coated with Matrigel at 2 × 105 cells/0.5 mL per insert, and cultured under standard condition (5% CO2 with balanced air). Forty-eight hours later, cells were fixed and stained with Giemsa stain. Non-invaded cells on the upper surface of the membrane were removed using a cotton swab. The number of stained cells at the lower surface of the membrane was counted in at least 10 randomly selected non-overlapping fields by light microscopy. JEG-3 cells cultured at 2% O2 with 5% CO2/balanced N2 but without siRNA transfection throughout the experiments were used as the positive controls. All experiments were performed in triplicate and the invasion activity was expressed as the percentage of invaded cell number compared to the corresponding control.
Real-time quantitative PCR
Real-time quantitative PCR analysis was performed as previously described [10]. Assay-on-Demand TaqMan primers and probes for human BECN1 (Hs00186838_m1), LC3B (Hs00797944_s1), and DRAM1 (Hs00218048_m1) were obtained from Applied Biosystems (Life Technologies, Grand Island, NY, USA); 18S ribosomal RNA (Hs99999901_s1) was used as an endogenous control. Thermal cycling was initiated via 2 min of incubation at 50°C, followed by an initial denaturation step of 10 min at 95°C, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. All samples were analyzed on the same run, and each sample was run in triplicate. Relative quantities of BECN1, LC3B, DRAM, and 18S ribosomal RNA were calculated using the comparative threshold cycle method.
Immunofluorescence
Immunofluorescence was used to determine the cellular localization of beclin-1, LC3B, DRAM, and cytokeratin 7 in the placenta at early and late gestation, as previously described [4]. Briefly, after blocking of any non-specific binding, 5-µm-thick cryosections were incubated with the following primary antibodies: rabbit anti-human beclin-1 (1:50; catalogue no. PD017, Medical and Biological Laboratories, Nagoya, Japan), rabbit anti-human LC3B (1:50; catalogue no. 2775S, Cell Signaling, Danvers, MA, USA), rabbit anti-human DRAM (1:100; catalogue no. ab68987, Abcam, Cambridge, UK) polyclonal antibodies, and mouse anti-human cytokeratin 7 monoclonal antibody (clone OV-TL12/30, 1:500; DakoCytomation, Glostrup, Denmark) at 4°C overnight. After the sections were washed, they were incubated with a cocktail of Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 594-conjugated goat anti-mouse IgG (10 µg/mL; Molecular Probes; Life Technologies, Grand Island, NY, USA) at room temperature for 1 h, mounted with Vectashield-DAPI (Vector Laboratories, Burlingame, CA, USA), and observed using a Leica TCS-SP2 confocal microscope (Leica Microsystems, Manheim, Germany). The negative controls used nonimmune rabbit IgG or mouse isotypic IgG instead of the primary antibody.
Western blot
Western blotting was performed as previously detailed [4]. After individual experiments, 50 to 100 µg of cytosolic protein was separated by 12% or 16% SDS-PAGE, transferred to nitrocellulose membranes, and probed with primary antibodies against human beclin-1 (1:500, catalogue no. 3738S, Cell Signaling), LC3B (1:500, Cell Signaling), and DRAM (2 µg/mL, catalogue no. SC-98654, Santa Cruz) at 4°C overnight. The relative intensities of protein signals were normalized to the corresponding β-actin (clone AC-15, 1:10000 dilution; Sigma) density and quantified by densitometric analysis using Image J software (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/).
Transmission electron microscopy
Autophagy of villous tissues was confirmed by transmission electron microscopy as previously described [4].
Statistical Analysis
Data are presented as the mean ± S.D. or median and interquartile range when data were not normally distributed. Data were analyzed and plotted using Prism 5 for Mac OS X, version 5.0d (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis was computed using 1-way analysis of variance (ANOVA) followed by Bonferroni’s test or the Kruskal-Wallis test followed by Dunn’s multiple comparison test. In experiments studying the effect of oxygen and glucose on autophagic changes in primary cytotrophoblast cells, comparisons between groups were undertaken by 2-way ANOVA with oxygen and glucose as the main factors and oxygen × glucose as the interaction term. P-values < 0.05 were considered to be statistically significant.
Results
Levels of the autophagy-related proteins beclin-1, DRAM, and LC3B in villous tissues throughout gestation
Using transmission electron microscopy, autophagic vacuoles were clearly identified in the trophoblast layer of villous tissues from early and late gestation (Figure 1).
Figure 1. Ultrastructural assessment of autophagic vacuoles in the trophoblast layer of placentas from early and late gestation.

Electron micrographs illustrating autophagic vacuoles in the villous cytotrophoblast at 8 weeks of gestation (A) and in the syncytiotrophoblast at 39 weeks of gestation (B). Scale bar = 1 µm and 250 nm in insets.
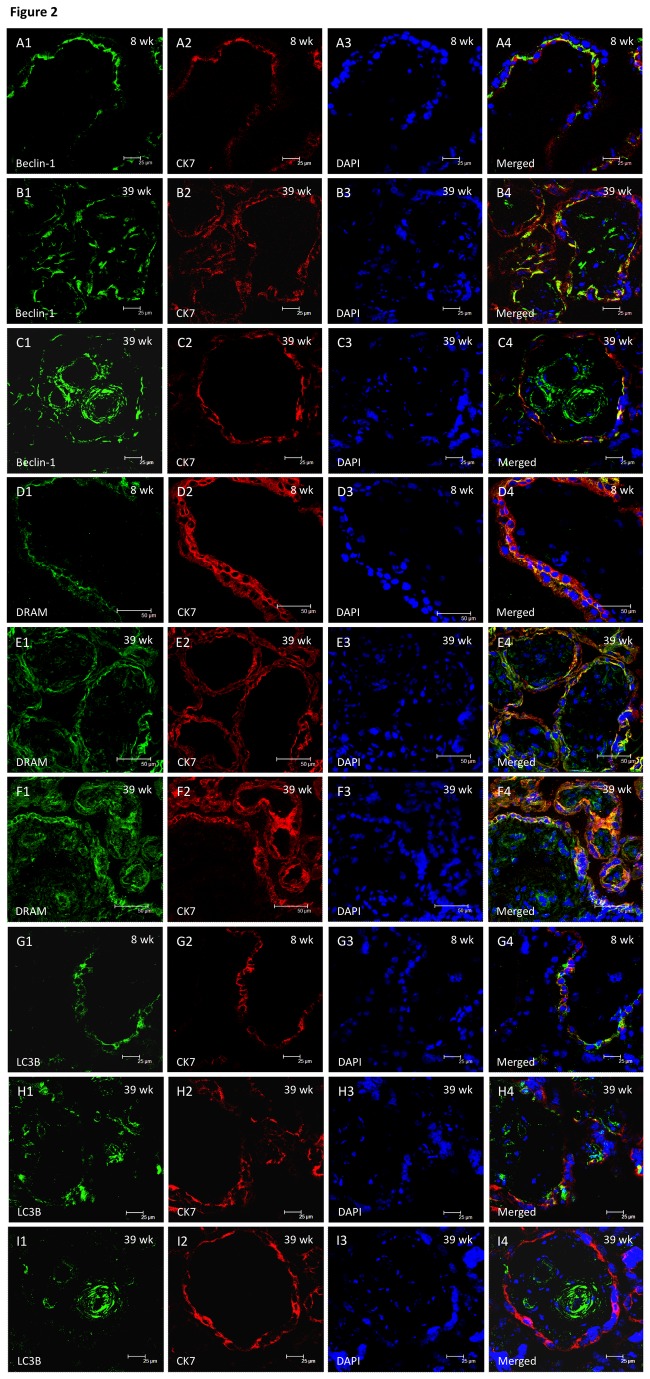
We next studied changes in 3 autophagy-related proteins, namely beclin-1, DRAM, and LC3B, to evaluate autophagic changes in placentas throughout gestation. Cellular localization of beclin-1, DRAM, and LC3B was demonstrated by immunofluorescence (Figure 2). Formation of beclin-1 and LC3B punctae was observed in the trophoblast layer of villous tissues of early and late gestation (Figures 2A1-2C4 and 2G1-H4), but also in the villous endothelium and adjacent smooth muscle cells, and some stromal cells in villous tissues of late gestation (Figures 2C1-C4 and 2I1-2I4). Immunoreactivity of DRAM was mainly localized in trophoblast cells at early and late gestation (Figures 2D1-2E4). In villous tissues of late gestation, immunofluorescence of DRAM was also noted in some stromal cells and villous endothelium (Figures 2F1-F4).
Figure 2. Cellular localization of beclin-1, DRAM, and LC3B in villous tissues from early and late gestation by immunofluorescence.
Formation of beclin-1 and LC3B punctae was observed in the trophoblast layer of villous tissues of early and late gestation (A1-C4 and G1-H4), but also in the villous endothelium and adjacent smooth muscle cells, and some stromal cells in villous tissues of late gestation (C1-C4 and I1-I4). Immunoreactivity of DRAM was mainly localized in trophoblast cells at early and late gestation (D1-E4). In villous tissues of late gestation, immunofluorescence of DRAM was also noted in some stromal cells and villous endothelium (F1-F4). The sections were stained with cytokeratin 7 and DAPI to highlight trophoblast cells and nuclei, respectively. Scale bar = 25 μm (A1-C4, G1-I4) and 50 μm (D1-F4).
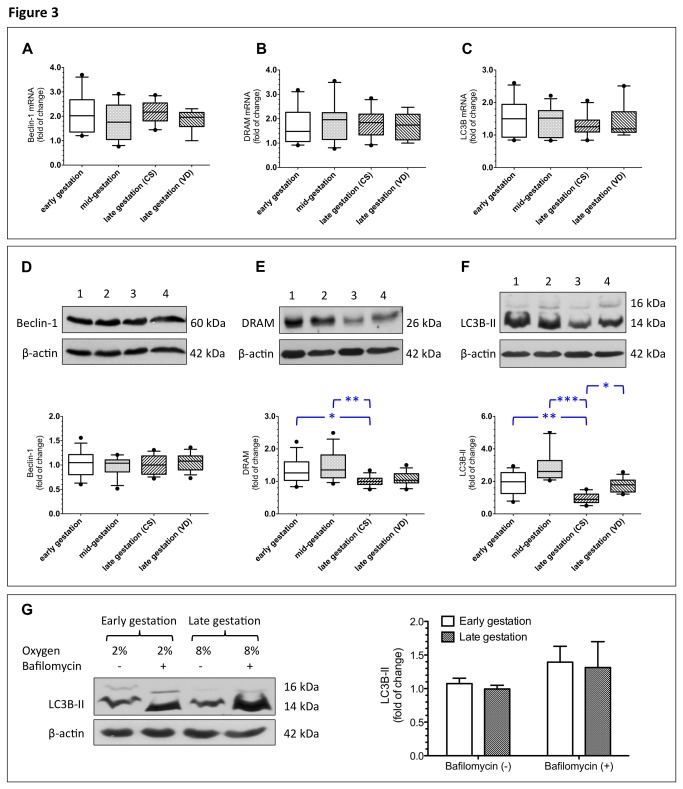
By analyzing a total of 40 samples representing different gestational stages, we found that BECN1, DRAM, and LC3B mRNAs were continuously expressed in the villous tissues throughout gestation and there were no significant differences in the levels of BECN1, DRAM, and LC3B mRNA among villous tissues of different stages of gestations and among women with different modes of delivery (Figures 3A–3C).
Figure 3. Beclin-1, DRAM, and LC3B levels and autophagic flux in villous tissues from various stages of gestation and different modes of delivery.
In a total of 40 samples representing different gestational stages and modes of delivery, BECN1, DRAM, and LC3B mRNA were continuously expressed in villous tissues throughout gestation and there were no significant differences in the levels of BECN1, DRAM, and LC3B mRNA between villous tissues of different stages of gestations and between women with different modes of delivery (A–C). On the other hand, levels of beclin-1 remained constant throughout gestation (D), while significantly higher levels of DRAM and LC3B-II were noted in villous tissues from early and mid-gestation than in those of term placentas obtained from elective CS (E–F). Furthermore, women with VD had higher levels of LC3B-II than did those with CS (F). CS, cesarean section; VD, vaginal delivery. A total of 10 placentas from early gestation (7–12 weeks), 10 from mid-gestation (13–28 weeks), 10 from late gestation with CS (38–39 weeks), and 10 from late gestation with VD (38–40 weeks) were used for analysis. β-actin was used to normalize loading variability. Lane 1, villous tissues from early gestation; lane 2, villous tissues from mid-gestation; lane 3, villous tissues from late gestation with CS; and lane 4, villous tissues from late gestation with VD. Data are presented as median and interquartile range and plotted as box and whisker plots (box = interquartile range, whiskers = 90th and 10th percentiles). *P < 0.05, **P < 0.01, and ***P < 0.001, compared to villous tissues of late gestation with CS (A-F). Villous explants prepared from early gestation and from term pregnancy were cultured under 2% and 8% oxygen concentrations, respectively, with or without bafilomycin A1. Villous explants from early and late gestation showed similar levels of LC3B-II when exposed to bafilomycin A1, indicating that these two groups of tissues had comparable rates of autophagic flux under physiological oxygen concentrations. Data are presented as mean ± S.D. from 5 individual experiments (G).
Conversely, levels of beclin-1 remained constant throughout gestation, while significantly higher levels of DRAM and LC3B-II were noted in villous tissues from early and mid-gestation than in those from late gestation with CS (Figures 3D-3F). Moreover, women with VD showed higher levels of LC3B-II in villous tissues than did those with CS (Figure 3F).
Autophagic flux in villous tissues from early and late gestation
Decreased LC3B-II levels in villous tissues of late gestation with CS can be associated with either reduced autophagosome synthesis or increased autophagosome turnover by lysosomal degradation [6]. To uncouple these effects, villous explants prepared from early and late gestation were cultured under 2% and 8% oxygen concentrations, respectively, with or without bafilomycin A1. Bafilomycin A1 is an inhibitor of autophagosome content degradation. As shown in Figure 3G, villous tissues from early and late gestation showed similar levels of LC3B-II when exposed to bafilomycin A1, indicating that these two groups of tissues had comparable rates of autophagic flux under physiological oxygen concentrations. These results also suggest that increased degradation of LC3B-II in the autophagosome in villous tissues of late gestation than in those of early gestation.
Autophagy and cytotrophoblast cell viability and invasion activity
To further investigate the influence of autophagy on trophoblast cell behavior, we examined cell proliferation and invasion in JEG-3 cells transfected with specific siRNAs against BECN1, DRAM, and LC3B (Figure 4). As shown in Figure 4B, there was no difference in cell viability between cells transfected with specific siRNAs and those transfected with control siRNAs. For the invasiveness, increased invasion of JEG-3 cells was noted under 2% oxygen concentration compared to the standard condition (Figure 4C); however, there was no difference in the invasion activity under standard condition between cells transfected with siRNA against BECN1, DRAM, or LC3B, and cells transfected with control siRNA (Figures 4C and 4D).
Figure 4. The effects of reduced autophagy on viability and invasion activity of JEG-3 cells.
(A) Representative immunoblots for beclin-1, DRAM, and LC3B-II from JEG-3 cells transfected with or without specific siRNAs are shown. β-actin was used to normalize loading variability. Lane 1, cells transfected with control siRNAs; lane 2, cells transfected with BECN1 siRNA; lane 3, cells transfected with DRAM siRNA; and lane 4, cells transfected with LC3B siRNA. There were no differences in viability (B) and invasion activity (D) under standard culture condition between cells transfected with control siRNAs and cells transfected with specific siRNAs. JEG-3 cells cultured at 2% O2 with 5% CO2/balanced N2 but without siRNA transfection throughout the experiments were used as the positive controls for the invasion assay. Scale bar = 100 μm (C). Data are presented as mean ± S.D. A total of 5 individual experiments were performed (D).
The effects of oxygen-glucose deprivation on levels of beclin-1, DRAM, and LC3B mRNA and protein in primary cytotrophoblast cells
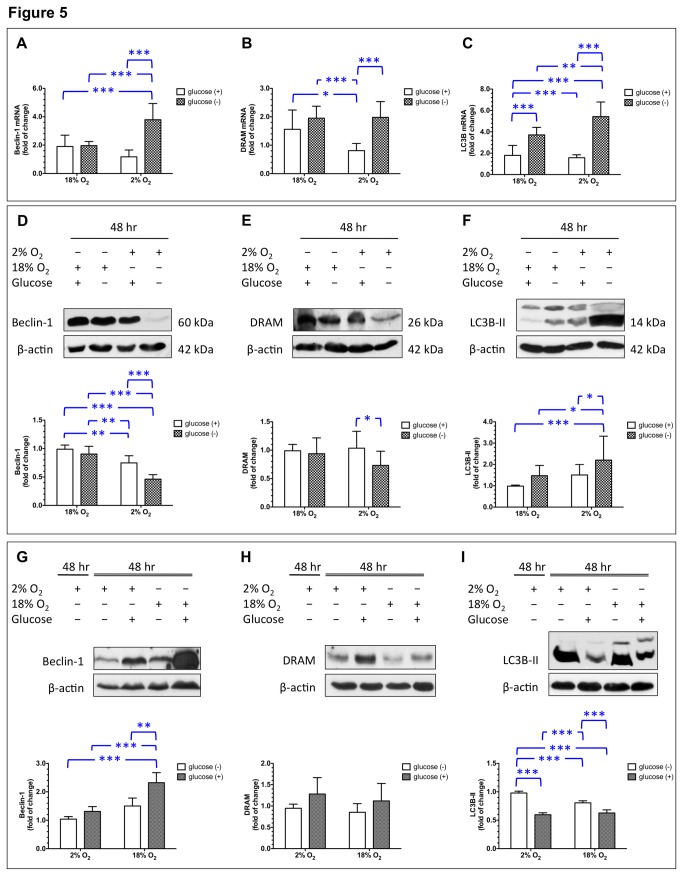
In pregnancy complications such as preeclampsia and FGR, perfusion of the intervillous space is reduced and becomes more variable due to defective transformation of the maternal spiral arteries [12,13]. We therefore studied the effects of reduced oxygen and glucose levels on autophagy in primary cytotrophoblast cells. As shown in Figures 5A and 5B, both oxygen and glucose deprivation had a statistically significant effect on mRNA levels of BECN1 (ANOVA oxygen effect, P < 0.05; ANOVA glucose effect, P < 0.001) and DRAM (ANOVA oxygen effect, P < 0.05; ANOVA glucose effect, P < 0.001); however, changes in BECN1 and DRAM transcription in cells cultured with supplemented glucose differed from those in cells under glucose deprivation conditions, depending on the oxygen concentration [ANOVA oxygen × glucose interaction effect, F(1,36) = 28.7, P < 0.0001 for BECN1 and ANOVA oxygen × glucose interaction effect, F(1,36) = 6.0, P < 0.05 for DRAM]. In 18% oxygen, cells under glucose deprivation conditions showed similar levels of BECN1 and DRAM mRNA as cells supplemented with glucose. In contrast, glucose deprivation led to significantly higher levels of beclin-1 and DRAM mRNA compared to glucose supplementation in 2% oxygen (P < 0.001).
Figure 5. Effects of deprivation and re-supplementation of oxygen and glucose on levels of beclin-1, DRAM, and LC3B in primary cytotrophoblast cells.
Cytotrophoblasts were cultured in a medium with or without glucose in 2% or 18% oxygen with 5% CO2/balanced N2 for 48 h. Data are presented as mean ± S.D. A total of 10 individual experiments were performed. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (A–F). Cytotrophoblasts were cultured in a medium without glucose in 2% O2/5% CO2/balanced N2 for 48 h and then subjected to 2% or 18% oxygen with or without glucose for another 48 h. Data are presented as mean ± S.D. A total of 10 individual experiments were performed. **, P < 0.01 and ***, P < 0.001 (G–I).
Similarly, both oxygen and glucose deprivation had a significant effect on the levels of LC3B mRNA (Figure 5C, ANOVA oxygen effect, P < 0.05; ANOVA glucose effect, P < 0.001), but changes in LC3B transcription in cells cultured with glucose supplementation differed from those in cells with glucose deprivation, depending on the oxygen concentration [ANOVA oxygen × glucose interaction effect, F(1,36) = 11.2, P < 0.01]. Specifically, cytotrophoblast cells treated with glucose deprivation had significantly higher levels of LC3B mRNA than did cells cultured with glucose supplementation, with either 18% or 2% oxygen (P < 0.001). Furthermore, the effect of glucose deprivation on the induction of LC3B transcription was more profound under 2% oxygen than under 18% oxygen (P < 0.01).
Figures 5D–5F show the effects of OGD on the changes of these three autophagy-related proteins, which were not consistent with the changes in mRNA levels. Statistical analysis demonstrated that both oxygen and glucose deprivation had a significant effect on the levels of beclin-1 (Figure 5D, ANOVA oxygen effect, P < 0.001; ANOVA glucose effect, P < 0.001), but these changes in cells cultured with glucose supplementation differed from those in cells cultured with glucose deprivation, depending on the oxygen concentration [ANOVA oxygen × glucose interaction effect, F(1,20) = 5.0, P < 0.05]. With 18% oxygen, cells treated with glucose deprivation had similar levels of beclin-1 as cells supplemented with glucose. In contrast, glucose deprivation led to significantly lower levels of beclin-1 than did glucose supplementation under 2% oxygen (P < 0.001).
For DRAM, statistical analysis demonstrated that only glucose deprivation had a significant effect on the levels of DRAM (Figure 5E, ANOVA glucose effect, P < 0.05), but did not show an oxygen × glucose interaction effect [F(1,20) = 0.08, P = 0.79]. In 18% oxygen, cells treated with glucose deprivation had similar levels of DRAM as cells supplemented with glucose. However, glucose deprivation led to significantly lower levels of DRAM than did glucose supplementation in 2% oxygen (P < 0.05).
Oxygen and glucose deprivation resulted in a different pattern of change for LC3B-II compared to the patterns of changes in the levels of beclin-1 and DRAM (Figure 5F). Specifically, both oxygen and glucose deprivation led to an increase in LC3B-II level (ANOVA oxygen effect, P < 0.01; ANOVA glucose effect, P < 0.001); however, changes in LC3B-II levels were different for cells cultured with glucose supplementation and those cultured with glucose deprivation, depending on the oxygen concentration [ANOVA oxygen × glucose interaction effect, F(1,20) = 25.5, P < 0.001]. Cytotrophoblast cells treated with glucose deprivation showed significantly higher levels of LC3B-II than did cells cultured with glucose supplementation, though a more profound effect was noted with 2% oxygen than with 18% oxygen (P < 0.05).
The effects of oxygen and glucose supplementation on levels of beclin-1, DRAM, and LC3B-II in primary cytotrophoblast cells after OGD
We next set to study the effects of reoxygenation and re-supplementation of glucose on levels of autophagic changes in primary cytotrophoblast cells after 48 h of OGD. As shown in Figure 5G, supplementation of oxygen and glucose led to a statistically significant increase in the levels of beclin-1 in cytotrophoblast cells after OGD treatment (ANOVA oxygen effect, P < 0.001; ANOVA glucose effect, P < 0.001), and a significant interaction effect between oxygen and glucose supplementation [ANOVA oxygen × glucose interaction effect, F(1,20) = 5.0, P < 0.05]. Supplementation of oxygen and glucose caused significantly increased beclin-1 levels compared to the levels in OGD controls (P < 0.001), and compared to the levels in cells supplemented with glucose (P < 0.001) or oxygen (P < 0.01) alone. For DRAM, supplementation of glucose after OGD, either with or without reoxygenation, seemed to increase the levels of DRAM (Figure 5H, ANOVA glucose effect, P = 0.03). However, there were no significant differences in the levels of DRAM between different groups, and considerable variations were noted between individual experiments.
In contrast, the effect of oxygen and glucose supplementation after OGD on LC3B-II levels was different from that on levels of beclin-1 and DRAM (Figure 5I). Statistical analysis revealed that supplementation of oxygen and glucose led to a significant decrease in LC3B-II levels in cytotrophoblast cells after OGD treatment (ANOVA oxygen effect, P < 0.01; ANOVA glucose effect, P < 0.001), but resulted in a significant interaction effect between oxygen and glucose supplementation [ANOVA oxygen × glucose interaction effect, F(1,20) = 25.5, P < 0.001]. Compared to OGD controls, cytotrophoblast cells supplemented with glucose or oxygen only, or both, showed significantly lower levels of LC3B-II (all P < 0.001). In addition, supplementation of glucose had a more profound effect on the reduction of LC3B-II levels than did reoxygenation.
Discussion
In this study, autophagy was clearly demonstrated by transmission electron microscopy and immunofluorescence in the placentas at early and late gestation. Furthermore, BECN1, DRAM, and LC3B were consistently expressed in the placentas, though no significant differences in protein levels of these molecules were noted in placentas between early and mid-gestation, and late gestation with VD. Together, these results indicate that autophagy is important in the development of the human placenta.
The biological significance of autophagy in the human placenta remains unclear. One possible role of autophagy is to protect trophoblasts from apoptosis induced by stresses such as hypoxia or nutritional deprivation. The human placenta is hemochorial; maternal blood comes in direct contact with the chorion [14]. Therefore, the villous trophoblasts are easily exposed to stresses created by variable maternal blood flow. In pregnancy complications such as preeclampsia and FGR, perfusion of the intervillous space is reduced and becomes more variable due to defective transformation of the maternal spiral arteries by endovascular invasion of the extravillous cytotrophoblasts. Such profound changes in oxygen and nutritional supply may cause dysfunction of mitochondria and endoplasmic reticulum and lead to apoptosis of trophoblasts [12,13]. To avoid widespread dysregulated apoptosis, we hypothesize that the autophagic pathway is activated to maintain cellular homeostasis, thus allowing trophoblast cells to survive oxygen and glucose deprivation caused by intermittent perfusion of the intervillous space. In support of this hypothesis, increased autophagy in cytotrophoblasts, as depicted by a significant increase in the levels of LC3B-II, was noted after OGD treatment compared to the observation in cytotrophoblasts kept in standard culture conditions. Moreover, increased autophagy was found in placentas from women with severe preeclampsia and FGR compared with normal pregnant women [4,5].
Autophagy has also been implicated to be involved in trophoblast invasion [15], though the results are conflicting. Nakashima and co-workers found that autophagy induced by hypoxia enhanced the invasion capacity of extravillous trophoblast (EVT) cell lines [16]. In contrast, Choi and colleagues knocked down hypoxia-inducible factor (HIF)-1α with specific siRNA and showed that decreased HIF-1α was associated with increased autophagy but reduced invasion activity of HTR-8/SV-neo, an EVT cell line, under both normoxia and hypoxia [17]. Here, we found that there were no differences in viability and invasion activity under standard culture condition between JEG-3 cells transfected with specific siRNA against BECN1, DRAM, or LC3B and cells transfected with control siRNA. Further studies are therefore needed to clarify the role of autophagy in trophoblast invasion.
The molecular events underlying regulation of autophagy in human placenta have yet to be fully elucidated. The HIF-1α-associated pathway has been found to be involved in the activation of autophagy induced by hypoxia [17,18]. Our observation of a more profound impact of OGD on autophagic changes than hypoxia alone suggests the presence of mechanisms other than the HIF-1-mediated pathway in the regulation of autophagy in trophoblasts. The mammalian target of rapamycin (mTOR) pathway is also important in the regulation of autophagy [18]. The mTOR pathway integrates nutritional status and stress signals, and regulation of amino acid transportation by glucose via the mTOR signaling has been observed in cultured primary cytotrophoblast cells [19]. Recently, Chen and colleagues reported that reduced mTOR activity was associated with an increased autophagic flux in primary cytotrophoblast cells [9]. Indeed, both amino acid transporter and mTOR activity are reduced in pregnancies complicated by FGR [20,21], a condition associated with increased autophagy [3,4].
In this study, we found that OGD caused a significant increase in the levels of BECN1, DRAM, and LC3B mRNA; however, the levels of beclin-1 and DRAM were lower than the levels in standard culture conditions. The discrepancy between the changes of mRNA and protein may be caused by inhibition of protein synthesis during OGD. Protein synthesis consumes a great deal of ATP. Adaptation to lowering oxygen and glucose levels requires coordinated downregulation of metabolic demand and supply to prevent a mismatch in ATP utilization and production that might culminate in a bioenergetic collapse. OGD diminishes ATP utilization by inhibiting mRNA translation and the activity of the Na-K-ATPase, and with inhibition of multiple mechanisms including mTOR signaling pathway [22]. Therefore, levels of beclin-1 and DRAM decreased during OGD, but increased when oxygen and glucose were restored. Our results also show differential changes in beclin-1, DRAM, and LC3B levels in response to changes in oxygen and glucose concentrations, indicating the complexity of interactions between these autophagy-related proteins.
In summary, our results indicate that autophagy is involved in the development of the human placenta, and that alterations in oxygen and glucose levels may participate in the regulation of autophagic changes in cytotrophoblast cells. We speculate that autophagy is important in the protection of trophoblast cells from injury caused by deficits in oxygen and glucose during pregnancy.
Funding Statement
This work was supported by the National Science Council, Taiwan (NSC 100-2314-B-182A-082) and Chang Gung Memorial Hospital (CMRPG100021, CMRPG100022) to THH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290: 1717-1721. doi: 10.1126/science.290.5497.1717. PubMed: 11099404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armant DR (2011) Autophagy's expanding role in development: implantation is next. Endocrinology 152: 1739-1741. doi: 10.1210/en.2011-0220. PubMed: 21511986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curtis S, Jones CJ, Garrod A, Hulme CH, Heazell AE (2013) Identification of autophagic vacuoles and regulators of autophagy in villous trophoblast from normal term pregnancies and in fetal growth restriction. J Matern Fetal Neonatal Med 26: 339-346. doi: 10.3109/14767058.2012.733764. PubMed: 23039021. [DOI] [PubMed] [Google Scholar]
- 4. Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL et al. (2012) Increased autophagy in placentas of intrauterine growth-restricted pregnancies. PLOS ONE 7: e40957. doi: 10.1371/journal.pone.0040957. PubMed: 22815878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH et al. (2008) Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci 15: 912-920. doi: 10.1177/1933719108319159. PubMed: 19050324. [DOI] [PubMed] [Google Scholar]
- 6. Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8: 445-544. doi: 10.4161/auto.19496. PubMed: 22966490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crighton D, Wilkinson S, Ryan KM (2007) DRAM links autophagy to p53 and programmed cell death. Autophagy 3: 72-74. PubMed: 17102582. [DOI] [PubMed] [Google Scholar]
- 8. Shen ZY, Li EM, Lu SQ, Shen J, Cai YM et al. (2008) Autophagic and apoptotic cell death in amniotic epithelial cells. Placenta 29: 956-961. doi: 10.1016/j.placenta.2008.09.001. PubMed: 18926571. [DOI] [PubMed] [Google Scholar]
- 9. Chen B, Longtine MS, Nelson DM (2012) Hypoxia induces autophagy in primary human trophoblasts. Endocrinology 153: 4946-4954. doi: 10.1210/en.2012-1472. PubMed: 22878401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hung TH, Chen SF, Li MJ, Yeh YL, Hsieh TT (2010) Differential effects of concomitant use of vitamins C and E on trophoblast apoptosis and autophagy between normoxia and hypoxia-reoxygenation. PLOS ONE 5: e12202. doi: 10.1371/journal.pone.0012202. PubMed: 20808946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung TH, Chen SF, Hsieh CC, Hsu JJ, Li MJ et al. (2008) Tumor necrosis factor-alpha converting enzyme in the human placenta throughout gestation. Reprod Sci 15: 195-209. doi: 10.1177/1933719107310709. PubMed: 18276953. [DOI] [PubMed] [Google Scholar]
- 12. Burton GJ (2009) Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215: 27-35. doi: 10.1111/j.1469-7580.2008.00978.x. PubMed: 19175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burton GJ, Jauniaux E, Charnock-Jones DS (2010) The influence of the intrauterine environment on human placental development. Int J Dev Biol 54: 303-312. doi: 10.1387/ijdb.082764gb. PubMed: 19757391. [DOI] [PubMed] [Google Scholar]
- 14. Burton GJ, Woods AW, Jauniaux E, Kingdom JC (2009) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473-482. doi: 10.1016/j.placenta.2009.02.009. PubMed: 19375795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saito S, Nakashima A (2013) Review: The role of autophagy in extravillous trophoblast function under hypoxia. Placenta 34 Suppl: S79-S84. doi: 10.1016/j.placenta.2013.06.233. PubMed: 23306070. [DOI] [PubMed] [Google Scholar]
- 16. Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T et al. (2013) Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy 9: 303-316. doi: 10.4161/auto.22927. PubMed: 23321791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi JH, Lee HJ, Yang TH, Kim GJ (2012) Effects of hypoxia inducible factors-1alpha on autophagy and invasion of trophoblasts. Clin Exp. Reprod Med 39: 73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehrpour M, Esclatine A, Beau I, Codogno P (2010) Overview of macroautophagy regulation in mammalian cells. Cell Res 20: 748-762. doi: 10.1038/cr.2010.82. PubMed: 20548331. [DOI] [PubMed] [Google Scholar]
- 19. Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T (2009) Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol 297: C723-C731. doi: 10.1152/ajpcell.00191.2009. PubMed: 19587219. [DOI] [PubMed] [Google Scholar]
- 20. Roos S, Jansson N, Palmberg I, Säljö K, Powell TL et al. (2007) Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582: 449-459. doi: 10.1113/jphysiol.2007.129676. PubMed: 17463046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I et al. (2008) Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 173: 451-462. doi: 10.2353/ajpath.2008.071193. PubMed: 18583310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wheaton WW, Chandel NS (2011) Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 300: C385-C393. doi: 10.1152/ajpcell.00485.2010. PubMed: 21123733. [DOI] [PMC free article] [PubMed] [Google Scholar]