Abstract
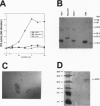
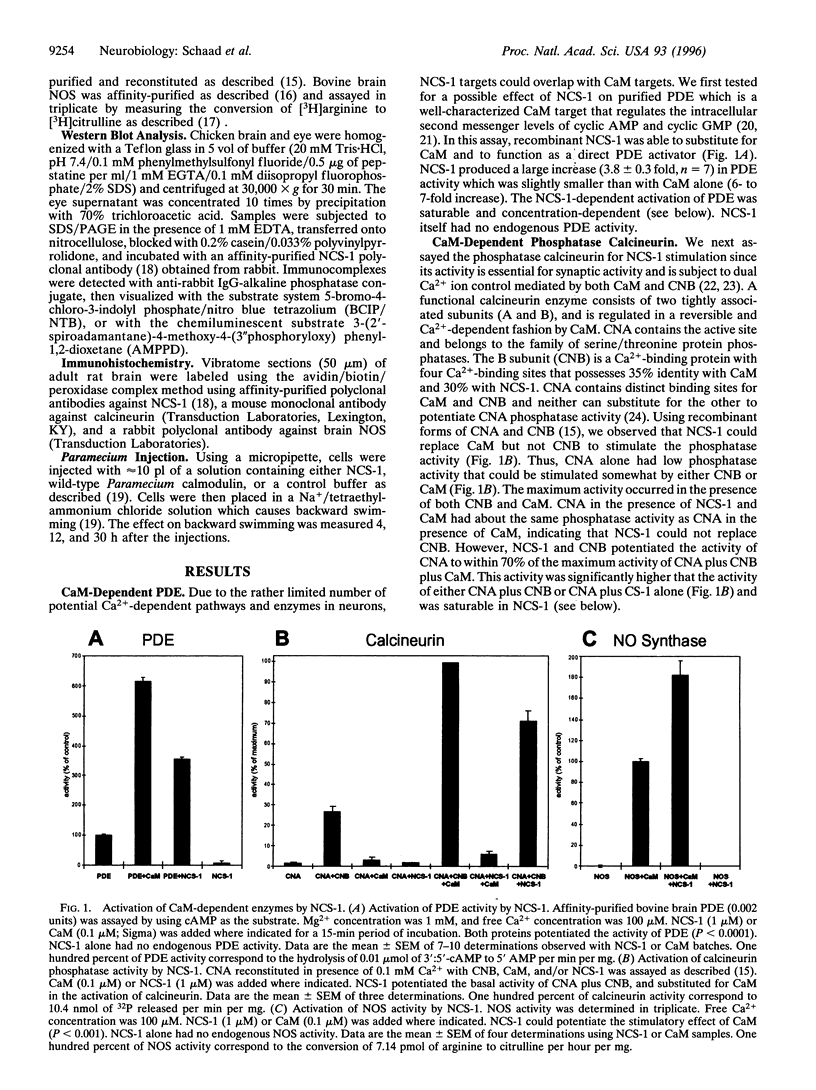
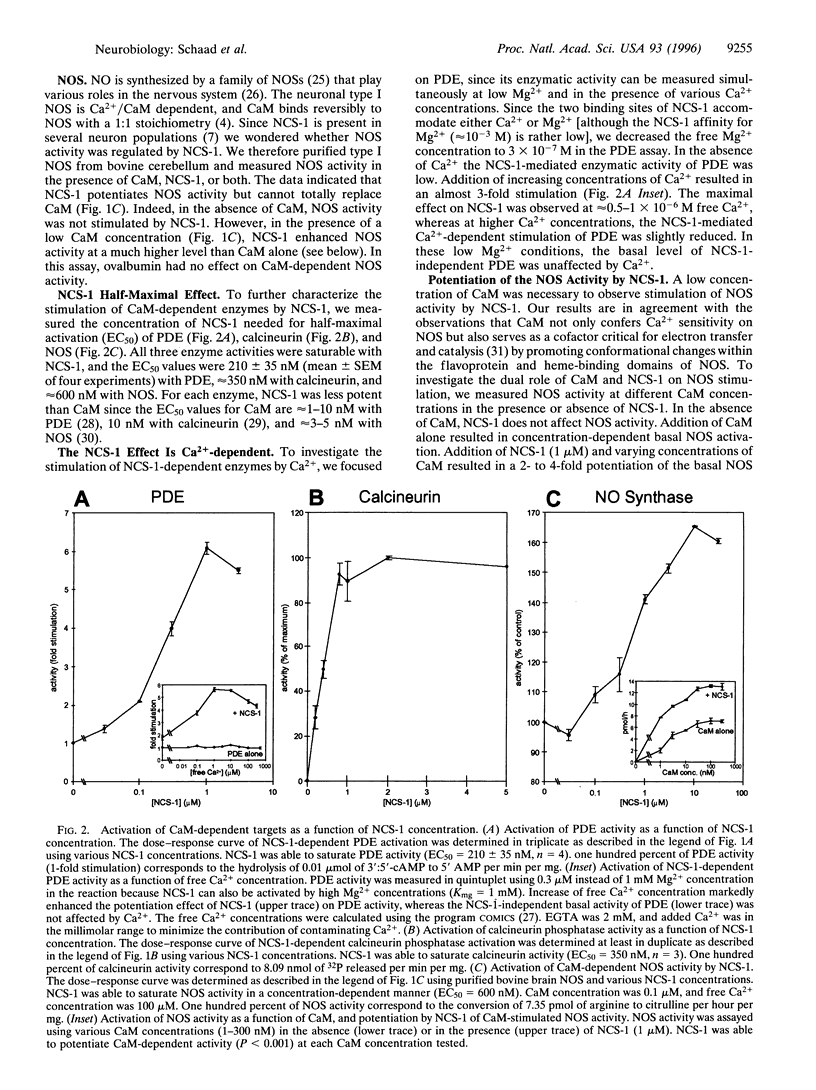
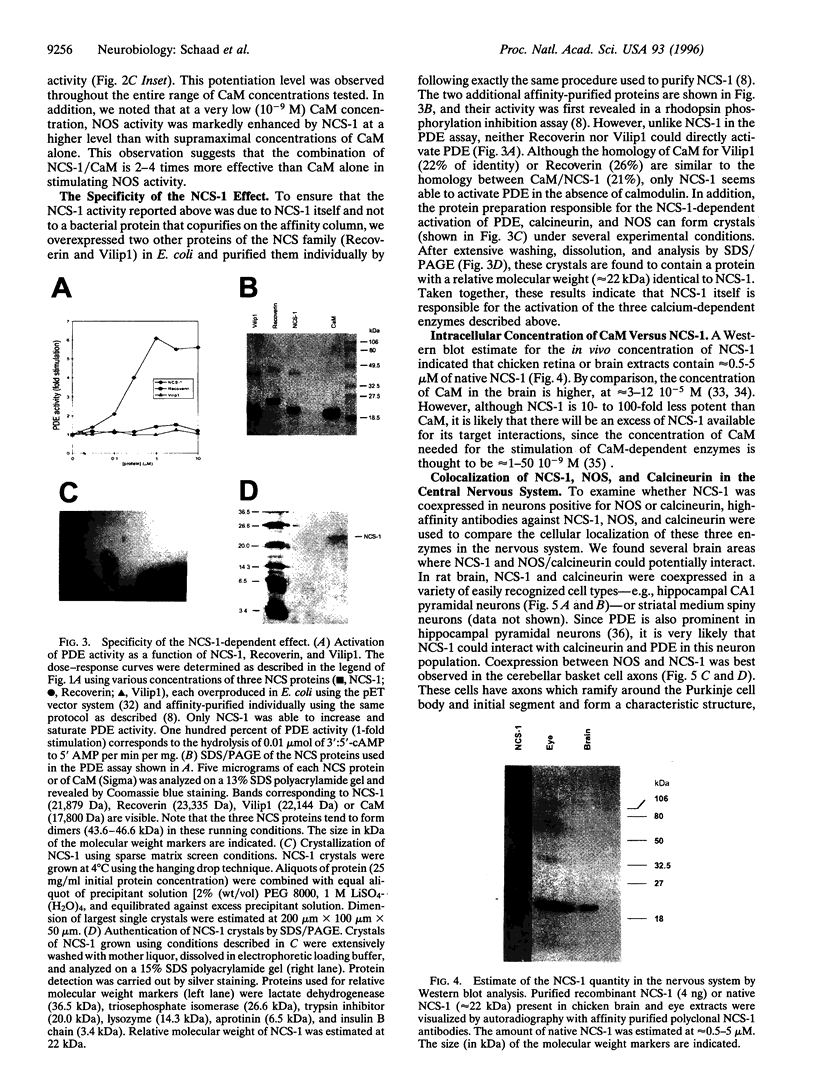
Ca2+ and its ubiquitous intracellular receptor calmodulin (CaM) are required in the nervous system, among a host of cellular responses, for the modulation of several important enzymes and ion channels involved in synaptic efficacy and neuronal plasticity. Here, we report that CaM can be replaced by the neuronal calcium sensor NCS-1 both in vitro and in vivo. NCS-1 is a calcium binding protein with two Ca(2+)-binding domains that shares only 21% of homology with CaM. We observe that NCS-1 directly activates two Ca2+/CaM-dependent enzymes (3':5'-cyclic nucleotide phosphodiesterase and protein phosphatase calcineurin). Co-activation of nitric oxide synthase by NCS-1 and CaM results in a higher activity than with CaM alone. Moreover, NCS-1 is coexpressed with calcineurin and nitric oxide synthase in several neuron populations. Finally, injections of NCS-1 into calmodulin-defective cam1 Paramecium partially restore wildtype behavioral responses. With this highly purified preparation of NCS-1, we have obtained crystals suitable for crystallographic structure studies. NCS-1, despite its very different structure, distribution, and Ca(2+)-binding affinity as compared with CaM, can substitute for or potentiate CaM functions. Therefore, NCS-1 represents a novel protein capable of mediating multiple Ca(2+)-signaling pathways in the nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Soud H. M., Stuehr D. J. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber A., Schmid G., Hempel K. Calmodulin content in specific brain areas. Exp Brain Res. 1984;56(2):323–326. doi: 10.1007/BF00236287. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Bender P., Snavely L., Rebhun L. I., Steward O. Distribution and subcellular localization of calmodulin in adult and developing brain tissue. Neuroscience. 1983 Oct;10(2):449–461. doi: 10.1016/0306-4522(83)90145-8. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Yau K. W. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994 Apr 7;368(6471):545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Durussel I., Comte M., Nef S., Nef P., Lenz S. E., Gundelfinger E. D. Cation binding and conformational changes in VILIP and NCS-1, two neuron-specific calcium-binding proteins. J Biol Chem. 1994 Dec 30;269(52):32807–32813. [PubMed] [Google Scholar]
- De Castro E., Nef S., Fiumelli H., Lenz S. E., Kawamura S., Nef P. Regulation of rhodopsin phosphorylation by a family of neuronal calcium sensors. Biochem Biophys Res Commun. 1995 Nov 2;216(1):133–140. doi: 10.1006/bbrc.1995.2601. [DOI] [PubMed] [Google Scholar]
- De Raad S., Comte M., Nef P., Lenz S. E., Gundelfinger E. D., Cox J. A. Distribution pattern of three neural calcium-binding proteins (NCS-1, VILIP and recoverin) in chicken, bovine and rat retina. Histochem J. 1995 Jul;27(7):524–535. doi: 10.1007/BF02388752. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Perrino B. A., Soderling T. R. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990 Feb 5;265(4):1924–1927. [PubMed] [Google Scholar]
- Hinrichsen R. D., Burgess-Cassler A., Soltvedt B. C., Hennessey T., Kung C. Restoration by calmodulin of a Ca2+-dependent K+ current missing in a mutant of Paramecium. Science. 1986 Apr 25;232(4749):503–506. doi: 10.1126/science.2421410. [DOI] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Huang C. Y., Chau V., Chock P. B., Wang J. H., Sharma R. K. Mechanism of activation of cyclic nucleotide phosphodiesterase: requirement of the binding of four Ca2+ to calmodulin for activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):871–874. doi: 10.1073/pnas.78.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard M. J., Klee C. B. Calmodulin binding by calcineurin. Ligand-induced renaturation of protein immobilized on nitrocellulose. J Biol Chem. 1987 Nov 5;262(31):15062–15070. [PubMed] [Google Scholar]
- Jarrett H. W., Madhavan R. Calmodulin-binding proteins also have a calmodulin-like binding site within their structure. The flip-flop model. J Biol Chem. 1991 Jan 5;266(1):362–371. [PubMed] [Google Scholar]
- Kawamura S., Hisatomi O., Kayada S., Tokunaga F., Kuo C. H. Recoverin has S-modulin activity in frog rods. J Biol Chem. 1993 Jul 15;268(20):14579–14582. [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C. Assay of cyclic nucleotide phosphodiesterase using radiolabeled and fluorescent substrates. Methods Enzymol. 1988;159:457–470. doi: 10.1016/0076-6879(88)59045-6. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Vaughan M. Purification and properties of calmodulin-activated cyclic nucleotide phosphodiesterase from mammalian brain. Methods Enzymol. 1988;159:557–573. doi: 10.1016/0076-6879(88)59054-7. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Maley M. E., Preston R. R., Ling K. Y., Wallen-Friedman M. A., Saimi Y., Kung C. Mutations in paramecium calmodulin indicate functional differences between the C-terminal and N-terminal lobes in vivo. Cell. 1990 Jul 13;62(1):165–174. doi: 10.1016/0092-8674(90)90250-i. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nakayama S. Evolution of EF-hand calcium-modulated proteins. IV. Exon shuffling did not determine the domain compositions of EF-hand proteins. J Mol Evol. 1993 May;36(5):477–488. doi: 10.1007/BF02406723. [DOI] [PubMed] [Google Scholar]
- Lenz S. E., Henschel Y., Zopf D., Voss B., Gundelfinger E. D. VILIP, a cognate protein of the retinal calcium binding proteins visinin and recoverin, is expressed in the developing chicken brain. Brain Res Mol Brain Res. 1992 Sep;15(1-2):133–140. doi: 10.1016/0169-328x(92)90160-d. [DOI] [PubMed] [Google Scholar]
- Liu M., Chen T. Y., Ahamed B., Li J., Yau K. W. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994 Nov 25;266(5189):1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- Merat D. L., Hu Z. Y., Carter T. E., Cheung W. Y. Bovine brain calmodulin-dependent protein phosphatase. Regulation of subunit A activity by calmodulin and subunit B. J Biol Chem. 1985 Sep 15;260(20):11053–11059. [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nef S., Fiumelli H., de Castro E., Raes M. B., Nef P. Identification of neuronal calcium sensor (NCS-1) possibly involved in the regulation of receptor phosphorylation. J Recept Signal Transduct Res. 1995 Jan-Mar;15(1-4):365–378. doi: 10.3109/10799899509045227. [DOI] [PubMed] [Google Scholar]
- Ni B., Rush S., Gurd J. W., Brown I. R. Molecular cloning of calmodulin mRNA species which are preferentially expressed in neurons in the rat brain. Brain Res Mol Brain Res. 1992 Mar;13(1-2):7–17. doi: 10.1016/0169-328x(92)90039-e. [DOI] [PubMed] [Google Scholar]
- Perrino B. A., Ng L. Y., Soderling T. R. Calcium regulation of calcineurin phosphatase activity by its B subunit and calmodulin. Role of the autoinhibitory domain. J Biol Chem. 1995 Jan 6;270(1):340–346. doi: 10.1074/jbc.270.1.340. [DOI] [PubMed] [Google Scholar]
- Ray S., Zozulya S., Niemi G. A., Flaherty K. M., Brolley D., Dizhoor A. M., McKay D. B., Hurley J., Stryer L. Cloning, expression, and crystallization of recoverin, a calcium sensor in vision. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5705–5709. doi: 10.1073/pnas.89.13.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y., Ling K. Y. Calmodulin activation of calcium-dependent sodium channels in excised membrane patches of Paramecium. Science. 1990 Sep 21;249(4975):1441–1444. doi: 10.1126/science.2169650. [DOI] [PubMed] [Google Scholar]
- Schaad N. C., Vanecek J., Schulz P. E. Photoneural regulation of rat pineal nitric oxide synthase. J Neurochem. 1994 Jun;62(6):2496–2499. doi: 10.1046/j.1471-4159.1994.62062496.x. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Seto-Ohshima A., Kitajima S., Sano M., Kato K., Mizutani A. Immunohistochemical localization of calmodulin in mouse brain. Histochemistry. 1983;79(2):251–257. doi: 10.1007/BF00489787. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Calmodulin and Ca2+-dependent phosphorylation and dephosphorylation of 63-kDa subunit-containing bovine brain calmodulin-stimulated cyclic nucleotide phosphodiesterase isozyme. J Biol Chem. 1986 Jan 25;261(3):1322–1328. [PubMed] [Google Scholar]
- Sikkink R., Haddy A., MacKelvie S., Mertz P., Litwiller R., Rusnak F. Calcineurin subunit interactions: mapping the calcineurin B binding domain on calcineurin A. Biochemistry. 1995 Jul 4;34(26):8348–8356. doi: 10.1021/bi00026a016. [DOI] [PubMed] [Google Scholar]
- Stemmer P. M., Klee C. B. Dual calcium ion regulation of calcineurin by calmodulin and calcineurin B. Biochemistry. 1994 Jun 7;33(22):6859–6866. doi: 10.1021/bi00188a015. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Datto G. A. Identification and characterization of a calmodulin-dependent nitric oxide synthase from GH3 pituitary cells. Biochem J. 1992 Jul 1;285(Pt 1):201–206. doi: 10.1042/bj2850201. [DOI] [PMC free article] [PubMed] [Google Scholar]