Abstract
Introduction
Enzymatic debridement is a method by which burn wounds can be prepared for coverage by skin grafts in patients presenting late. Many agents have been used in the past but none of them have been thoroughly evaluated. The present study was undertaken to assess the efficacy of Debridace, a commonly available debriding agent with papain and urea as its constituents.
Material and methods
A prospective descriptive study design was used to evaluate our experience. Almost symmetrical areas of the burnt surface were assessed and used for comparison. On one half of the wound, Debridace was applied while on the other silver sulphadiazine was used. The primary end point of this study was the extent of the achieved debridement at the end of the study period. Secondary outcomes were the presence of adverse effects such as pain and fever. All patients with sepsis were excluded from the study.
Results
The age of the subjects ranged from 9 to 80 years with an SD of 16. Large areas ranging from 5% to 20% body surface area with an SD of 4.27 were debrided by Debridace. Only two patients (3.33%) could complete the study. The rest of the recruited patients either had high fever (63.33%), excruciating pain (13.33%) or both (16.66%), which brought an end to enzymatic debridement.
Conclusion
Debridace, a papain–urea product, cannot be considered safe as an enzymatic debriding agent in its present form for use in major burn patients who present late with deep burn wounds that are large in size.
Keywords: Papain–urea, Enzymatic debridement, Burn wounds
Introduction
The current method of choice for burn debridement is early surgical excision as advocated by Janzekovic in 1970.1 However, patients reporting late who cannot be taken up for surgical excision need to be tackled differently. The eschar, made up of burned and traumatized tissue, is a hallmark of a burn wound. This prevents accurate assessment of the wound depth and may lead to the extension of injury to neighbouring tissue. The eschar also serves as a medium for bacterial growth, and consequent sepsis. As a result, prompt removal of the eschar is imperative to the healing of burns.2–5
While effective, surgical debridement has several major disadvantages. It can be non-selective and may sacrifice healthy surrounding tissues.6,7 Furthermore, surgical excision is painful and exposes patients to the risks of repeated anaesthesia and significant bleeding. Enzymatic debridement involves the application of exogenously derived proteolytic enzymes to a wound to accelerate a controlled digestion and removal of necrotic tissue with a potential to negate all the disadvantages of surgical debridement. However, the agents used have had several drawbacks. In particular, most enzymatic agents required prolonged and repeated exposures in order to achieve sufficient debridement, often necessitating further surgical or chemical debridement. Furthermore, repeated applications may result in local infection and promote sepsis.7–11
The most commonly available debriding agent that is cheap and an indigenous product is Debridace, a papain–urea combination. Papain, available in several enzymatic debriding agents, has been used to debride partial thickness burns. However, no controlled studies examining their effectiveness are available. The purpose of this study was to test the hypothesis that the administration of papain–urea ointment as enzymatic debridement to wounds that needed debridement would allow an autogenous split skin graft to be applied faster than with routine method.
Material and methods
A prospective descriptive study design was used to evaluate our experience with enzymatic debridement using papain–urea (Debridace is a cheap and commonly available preparation in the Indian market) in 30 consecutive major burn patients (>10% in children and >20% in adults of total body surface area involved) from May 2006 to May 2007. All patients were included in the study after obtaining a signed informed consent from the patients or from their legal guardians and the study was approved by the hospital ethical committee.
The study was conducted at the burn centre of a tertiary referral centre of the Armed Forces. Before embarking on the study, the agent (Debridace) was administered over small chronic wounds requiring debridement in five patients for testing efficacy of the enzymatic agent after they consented to be part of the trial. After encouraging results, the study patients were recruited. Patients were eligible for inclusion if they had major burns (>10% in children and >20% in adults of total body surface area involved) of deep second and third degree depth potentially requiring surgical debridement with patients reporting more than 5 days following burn injury and who were not taken up for early excision. More or less symmetrical areas of the burnt surface were assessed and used for comparison. 60% TBSA was used as cut off for the study beyond which the patients were not enrolled in the study.
Exclusion criteria were the following:
-
1.
Superficial burn wounds.
-
2.
Minor burn injury.
-
3.
Evidence of inhalation injury.
-
4.
Co-morbid conditions like diabetes mellitus, hypertension, cardiac disease including coronary events, concurrent acute injury or disease that might compromise the patient's life or welfare; significant haemotological, cardiovascular, hepatic or neoplastic diseases; or history of allergy, atopic disease, known hypersensitivity to papaya.
-
5.
Pregnant or lactating women.
-
6.
Patients in sepsis.
-
7.
Major burns with inadequate skin donor sites.
-
8.
Major burns >60% total body surface area.
-
9.
Patients unable to communicate grade of pain according to visual analogue scale.
Each burn wound was cleaned with saline soaked gauze. Reassessment of the burn wound depth was made and deep dermal or full thickness wounds divided into symmetric halves. On one half of the wound, Debridace was applied while on the other silver sulphadiazine was used. In case of contiguous burnt areas being assessed, the two were prevented from mixing by a gap of 3 cm between burn contiguous areas that was covered by saline soaked gauze as a barrier (Fig. 1). The ointment used was 30 g for every 10 cm × 10 cm (100 cm2 of surface area) of eschar. The patient was then closely observed for the presence of pain or itching and pain-relieving NSAID/narcotic were administered if required. The wounds were again inspected the next day and debridement assessed after wiping the area clean with gauze gently. Debridement was considered successful if a clean, bleeding surface was seen with no eschar. If debridement was not complete, the procedure was repeated. If the debridement was not complete after 10 days of application of Debridace, the procedure was abandoned and result considered a failure. During the enzymatic debridement, the patients were further monitored with twice weekly investigations and temperature charting as per burn centre protocol. The primary end point of this study was the extent of the achieved debridement at the end of the study period. Secondary outcomes were the presence of adverse effects such as pain and fever.
Fig. 1.

A patient with right half of upper trunk covered with Debridace while the opposite area is covered with silver sulphadiazine ointment. In between is saline soaked gauze as a barrier.
Visual assessment of debridement efficacy was determined by the senior co-worker by estimating the amount of original eschar that was removed according to the following classification:
Excellent (85–100%), good (70–84%), fair (60–69%) or poor (50–59%). Debridement of less than the original 49% of the eschar was considered a treatment failure. Photographic documentation of all burns was performed daily for confirmation of debridement.
All adverse events that occurred during hospitalization were recorded and their relationship to the treatment was judged according to their nature and timing in relation to the debridement. Based on past experience with other enzymatic debriding agents, assessment of fever and pain was especially important. Fever was defined as a temperature >38 °C (100.4 °F), and a rise in temperature of greater than 1 °C within 24 h of debridement was considered possibly related to the enzymatic debridement. Every rise in temperature of 1 °C (1.8 °F) was considered 1+. Enzymatic debridement was ended if the rise of the temperature was 2+. Pain was measured on a scale of 10 by the patient using the visual analogue scale and compared between areas where papain–urea ointment was used compared to silver sulphadiazine ointment as a control. The debridement was ended if pain was graded 8 or more on the scale or if the patient required narcotic analgesics to alleviate pain. The dressings were controlled by injectable narcotic for pain relief half hour before the dressing.
All investigations including haemogram, total leucocyte count, blood sugar, liver and renal function tests, serum electrolytes, routine urine examination, urine and blood cultures, serum electrolytes and swab cultures were done twice weekly. Antibiotics were administered as per culture reports. Sepsis was diagnosed when 5 or more of the following 9 parameters were present in one day:
-
1.
Obvious wound infection
-
2.
Positive blood culture
-
3.
Hypothermia (<35.5 °C), hyperthermia (>38.5 °C)
-
4.
Low (<3000/mm) or high (>15,000/cubmm) TLC
-
5.
Evidence of pneumonia or any localized abscess
-
6.
Development of petechial haemorrhages
-
7.
Confused and disoriented states
-
8.
Conversion of partial thickness burns to full thickness burns
-
9.
Paralytic ileus
The statistical analysis was carried out using SPSS 14.0. Mann–Whitney U test was used to test if any statistical difference existed between the pain scores of the control and the test groups.
Results
There were 34 patients who were recruited initially. However, four of them developed features of sepsis and were excluded from the study midway. Of the 30 patients studied, the age ranged from 9 to 80 years with an SD of 16. There were 12 males and 18 females with a male–female ratio of 1:1.5. Total body surface area burnt ranged from 20% to 60% with an average of 33.17%. Cause of burns was flame in 27 (90%) patients while it was scalds in the remaining 3 (10%). Percentage of burns over which Debridace was applied ranged from 5 to 20 with an SD of 4.27 (Table 1).
Table 1.
Patient profile.
| No. | Age | Sex | % of burns | Cause of burns | % of burns over which Debridace applied |
|---|---|---|---|---|---|
| 1 | 9 | F | 25 | Flame | 8 |
| 2 | 28 | F | 35 | Flame | 10 |
| 3 | 80 | F | 25 | Flame | 10 |
| 4 | 42 | F | 40 | Flame | 15 |
| 5 | 36 | F | 50 | Flame | 12 |
| 6 | 36 | M | 25 | Flame | 6 |
| 7 | 55 | F | 60 | Flame | 20 |
| 8 | 10 | F | 20 | Scalds | 5 |
| 9 | 48 | F | 20 | Flame | 6 |
| 10 | 36 | F | 35 | Flame | 10 |
| 11 | 49 | F | 25 | Flame | 10 |
| 12 | 39 | M | 30 | Flame | 8 |
| 13 | 34 | F | 35 | Flame | 12 |
| 14 | 36 | F | 25 | Flame | 6 |
| 15 | 37 | F | 25 | Flame | 8 |
| 16 | 42 | M | 30 | Flame | 8 |
| 17 | 48 | M | 40 | Flame | 15 |
| 18 | 39 | F | 60 | Flame | 20 |
| 19 | 41 | M | 45 | Flame | 18 |
| 20 | 45 | M | 35 | Flame | 12 |
| 21 | 12 | M | 50 | Flame | 14 |
| 22 | 19 | F | 60 | Flame | 18 |
| 23 | 28 | M | 25 | Flame | 10 |
| 24 | 14 | M | 30 | Flame | 8 |
| 25 | 17 | M | 20 | Flame | 8 |
| 26 | 54 | M | 25 | Flame | 10 |
| 27 | 47 | F | 20 | Scalds | 6 |
| 28 | 49 | M | 25 | Flame | 8 |
| 29 | 24 | F | 35 | Flame | 10 |
| 30 | 12 | F | 20 | Scalds | 6 |
Only two patients (3.33%) could complete the study period of 10 days. The rest of the recruited patients either had high fever (n = 19) (63.33%) (Figs. 2 and 3) or excruciating pain (n = 4) (13.33%) or both (n = 5) (16.66%) (Figs. 4–6) which brought a halt to enzymatic debridement (Table 2). Median score for pain in the control group was 3 while that for the test group was 7. There was a significant difference between the pain scores of the two groups (p = 0.000) with the test group experiencing more pain. Of the two patients who completed the study, one showed a poor (50%) result and another exhibited a good (70%) result after enzymatic debridement. They were both split skin grafted on the 20th and the 17th day of presentation respectively.
Fig. 2.
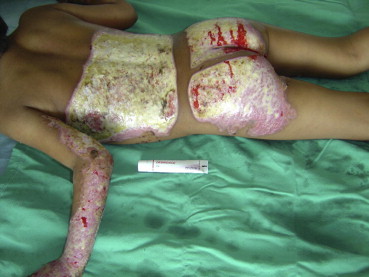
A child with wounds over the left of the back and buttocks before coverage with Debridace.
Fig. 3.
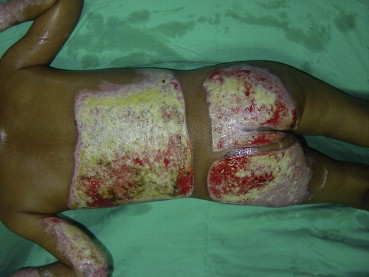
The same patient as in Fig. 2 after 5 days of debridement. The enzymatic debridement was discontinued the same day due to high fever.
Fig. 4.
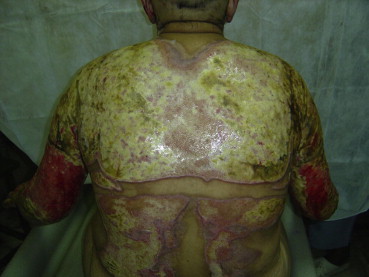
A patient with deep wounds over back and both upper limbs.
Fig. 5.
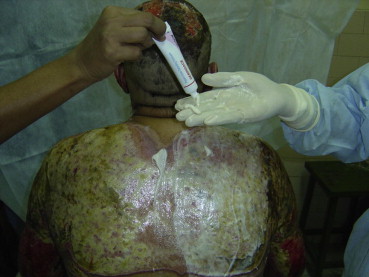
Same patient as in Fig. 4 with Debridace being applied over the right half of the back wound.
Fig. 6.
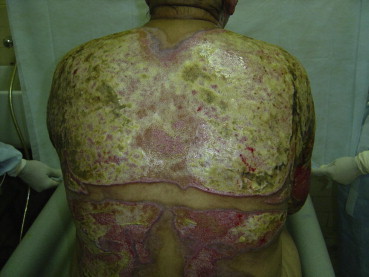
Same patient as in Figs. 4 and 5 after 2 days of debridement. The enzymatic debridement was discontinued the same day due to high fever and severe pain.
Table 2.
Results of enzymatic debridement.
| No | % of burns | % of burns over which Debridace applied | Site | Duration (days) | Result of enzymatic debridement | Fever | Pain |
|---|---|---|---|---|---|---|---|
| 1 | 25 | 8 | Back, buttocks | 5 | Equivocal | 2+ | 6 |
| 2 | 35 | 10 | Back, buttocks | 5 | Equivocal | 2+ | 7 |
| 3 | 25 | 10 | Neck, chest, right upper limb | 6 | Equivocal | 2+ | 7 |
| 4 | 40 | 15 | Neck, chest, right upper limb | 6 | Equivocal | 2+ | 6 |
| 5 | 50 | 12 | Abdomen, left thigh, left hand | 3 | Equivocal | 1+ | 8 |
| 6 | 25 | 6 | Left leg | 5 | Equivocal | 2+ | 6 |
| 7 | 60 | 20 | Right upper limb, right chest, abdomen | 4 | Equivocal | 2+ | 6 |
| 8 | 20 | 5 | Right upper limb | 5 | Equivocal | – | 8 |
| 9 | 20 | 6 | Abdomen, right thigh | 7 | Equivocal | 2+ | 6 |
| 10 | 35 | 10 | Back, right upper limb | 2 | Equivocal | 2+ | 8 |
| 11 | 25 | 10 | Back, right upper limb | 6 | Equivocal | 2+ | 7 |
| 12 | 30 | 8 | Left leg, left upper limb | 10 | Poor-50% | – | 7 |
| 13 | 35 | 12 | Abdomen, right thigh, right arm | 2 | Equivocal | 2+ | 8 |
| 14 | 25 | 6 | Right leg | 6 | Equivocal | – | 8 |
| 15 | 25 | 8 | Back, buttocks | 8 | Equivocal | 2+ | 6 |
| 16 | 30 | 8 | Back, buttocks | 10 | Good-70% | 1+ | 6 |
| 17 | 40 | 15 | Abdomen, left thigh, right upper limb | 2 | Equivocal | 2+ | 8 |
| 18 | 60 | 20 | Abdomen, right thigh, right upper limb | 2 | Equivocal | 2+ | 8 |
| 19 | 45 | 18 | Abdomen, right upper limb, right lower limb | 3 | Equivocal | 2+ | 7 |
| 20 | 35 | 12 | Back, buttocks | 5 | Equivocal | 2+ | 7 |
| 21 | 50 | 14 | Back, right posterior thigh | 5 | Equivocal | 2+ | 6 |
| 22 | 60 | 18 | Back, right upper limb, right lower limb | 5 | Equivocal | 2+ | 6 |
| 23 | 25 | 10 | Neck, chest, left upper limb | 6 | Equivocal | 2+ | 6 |
| 24 | 30 | 8 | Neck, chest, right upper limb | 7 | Equivocal | 2+ | 7 |
| 25 | 20 | 8 | Back, buttocks | 6 | Equivocal | 1+ | 8 |
| 26 | 25 | 10 | Abdomen, right thigh | 2 | Equivocal | 2+ | 8 |
| 27 | 20 | 6 | Right arm, forearm | 6 | Equivocal | 2+ | 7 |
| 28 | 25 | 8 | Left leg, left upper limb | 7 | Equivocal | 2+ | 6 |
| 29 | 35 | 10 | Neck, chest, left upper limb | 3 | Equivocal | 2+ | 7 |
| 30 | 20 | 6 | Right thigh | 6 | Equivocal | 2+ | 7 |
Discussion
Major burn injury is one of the most savage forms of trauma that can be sustained by human beings. Apart from causing possible death, it causes serious morbidity in the form of prolonged hospitalization, pain due to repeated dressings and an arduous rehabilitative process.
For healing to occur and recovery to take place, the burned skin must separate spontaneously or be removed, the resulting wounds must be covered, usually by skin grafts, the grafts must take, and the donor sites must heal. As long as dead skin is present or as long as the granulating area is unhealed, the possibility of serious infection, local and systemic, and subsequent death, exists.
Debridement is the cornerstone of burn wound management. There are four principal methods of debridement that are in current clinical use. These methodologies include autolytic, enzymatic, mechanical, biological and surgical or sharp debridement. Janzekovic in 1970 was the first to prove the necessity of an early operative treatment of deep burns.1 Since then, early excision and grafting have become the standard for the local treatment of those wounds which do not heal within 14 days, with a good final cosmetic and functional result. However, excision of these burn wounds can be done only if the patient reports early, usually before 5–7 days or before the contaminated wounds become infected. If the patients present later, the treatment of patients with major deep burns is fraught with complications such as sepsis and possible death and, delayed wound healing with the resultant sequel of fibro-proliferative scars such as hypertrophic scars or keloids. These patients require multiple surgical debridement which requires sedation or formal anaesthesia, there is pain of repeated dressings and blood transfusions abound. All these add to the misery of the patient as well as may jeopardize a favourable outcome in these patients. To overcome these drawbacks and to promote the eschar separation, enzymatic debridement has been tried throughout the world over many decades and have been considered by many as a potent alternative to surgical debridement.12–15 There have been many such agents that have been tried. Christopher Columbus used the pineapple juice to promote healing of burn wounds. Since then enzymes derived from micro-organisms such as clostridium histolyticum, collagenase, varidase, papain (from papaya) and bromelain (from pineapple) have been used. Many of these agents have been claimed to produce effective wound debridement after two to three applications, thus making the wound ready for grafting earlier than conservatively managed wounds. But the effectiveness of these enzymes has not yet been fully evaluated. Although enzymatic debridement would seem at first sight to be an attractive form of treatment, unfortunately the results are highly variable.8 The problem with a lot of these agents is that their efficacy and incidence of serious adverse reactions have not been evaluated. They have also not emerged as agents of standard of care in burn wound management as the results obtained have been patchy and inconsistent.
A papain–urea debriding ointment, Debridace, was used at our centre for enzymatic debridement. This was used as it is an indigenous preparation that is freely available and cost-effective. Papain is a proteolytic enzyme derived from the fruit of Carico, papaya, a potent digestant of non-viable tissue. Papain is relatively ineffective when used alone as a debriding agent and requires the presence of activators to stimulate its digestive potency. Urea, a small non-ionic molecule, is capable of interfering with disulphide bonds causing the protein to essentially relax.16 In addition, experimental evidence suggests urea may cause disruption of some of the disulfide bonds within proteins to expose particular thiol groups (–SH), which may serve as activators of papain.17 In Debridace, papain combines with urea, a denaturant of proteins to provide two supplemental chemical actions: (1) to expose the sulfhydryl groups (activators of papains) by solvent action, and (2) to denature the non-viable tissue in the lesions and render it more susceptible to enzymatic digestions. This combination produces a very effective debriding agent as demonstrated by in-vitro studies.18 Pharmacological studies have shown that the combination of papain and urea result in twice as much digestive activity as papain alone.
While small burn areas have been treated by many workers over decades,8,19–22 there is no work on enzymatic debridement in major burn areas except Garret23 who worked on fresh burns ranging from 3% to over 50% body surface area involvement and used sutilains. The enzyme treatment was restricted to 15% of body surface area or less, the main argument for this being to avoid the possibility that rapid lysis might stimulate invasion of the blood stream by bacteria from the wound. The present study was undertaken to evaluate the efficacy of enzymatic debridement in major burn wounds with enzyme treatment ranging from 5% to 20% body surface area.
Papain may be inactivated by heavy metal ions such as lead, mercury, and silver.24–26 Therefore, in our study, a gap of 3 cm between the two ointment bases was maintained to prevent the anti-microbial silver sulphadiazine interfering with the papain–urea debridement agent.
Once daily application was advocated by many workers including Guzman and Guzman21 and Burke and Golden.19 Debridace product description also promotes once or even twice daily application. In our study, once daily application was done.
Enzymatic debridement was satisfactory in the study by Guzman and Guzman by 48 h21 while it took on an average 21 days to be completed in the study by Burke and Golden with a varied range of 5–60 days.19 Debridement is usually complete with silver sulphadiazine alone by 2–3 weeks on an average. Therefore, for it to have statistical significance, the end point of enzymatic debridement was kept at 10 days of application of Debridace in the present study. Enzymatic debriding agents are typically used in conjunction with moist wound healing and serve as adjuncts to the autolytic debridement process.27 In our study too, a moist environment was used for dressing the wounds.
Enzymatic debridement would seem at first sight to be an attractive form of treatment. The necrotic tissue can be got rid of quickly after a thermal lesion, thus permitting early skin grafting of deep burns. The morbidity period is short, and the need for blood transfusion would appear to be reduced. Since only necrotic tissue is removed, early differentiation of partial thickness burns from full thickness burns should be possible. Unfortunately, the results of enzymatic debridement are highly variable. The process may still require several days to a few weeks to completely debride a wound, depending upon the agent chosen, the presentation of the devitalized tissue, and the skill with which the agent is employed. Because each preparation has its own particular protein digesting characteristics, it is important for the clinician to become familiar with the relative merits and shortcomings of these preparations as they apply to each necrotic wound condition. A number of factors could play a role here, such as the fact that thermal destruction of tissue need not always lead to the same type of damage. Hence, it is likely that different enzymes would be needed for debridement in different cases. Individual factors such as age, sex and race may also play a role.
Papain appears to have been considered a safe debriding agent. Clinical and laboratory experience has demonstrated that the enzyme does not harm the viable tissue surrounding the wound.21,28,29 In a study conducted in 1995, papain–urea ointment (with the trade name Accuzyme) was applied to 59 human subjects to evaluate the level of irritation and/or sensitization produced following multiple, repeated applications (10 applications per subject). No visible signs of erythema or oedema were noted for any Accuzyme-treated site relative to its corresponding untreated control site on any subject. Similarly, challenge testing conducted following a 14-day induction phase did not produce signs of sensitization in any of the subjects. The conclusion from this study was that Accuzyme did not indicate a potential for dermal irritation and/or sensitization.30 However, it is recommended that the results of the use of the papain/urea debriding system should not be extended to any other papain/urea debriding products without comparable controlled evaluation. Significant differences in carrier base, preservatives, the origin and activity level of the papain enzyme and in processing techniques could each be expected to contribute to different results for other papain/urea-based products. The debriding efficacy of the enzyme may depend on its delivery vehicle. The same enzyme formulated in different ointment bases under different manufacturing specifications could result in very different proteolytic activity (efficacy). The enzyme preparation used should have a constant composition, and it should be known which composition gives the best results.
In our study, the poor result of enzymatic debridement and high rate of adverse reactions, namely, pain and fever, could be a reflection of sub-optimal quality of preparation and inadequate standardization of the agent. It is also possible that the batch of the ointment used was inappropriate. However, based on the above results and in its present form, this agent cannot be considered safe for routine debridement of large burn wounds. Even though this study is suggestive, it is recommended that more research, especially a randomized controlled trial, may be directed towards re-evaluating the debriding agent.
Intellectual contribution of authors
Study concept: Col Vijay Langer, Col P.S Bhandari.
Drafting and manuscript revision: Col Vijay Langer, Brig S Rajagopalan.
Statistical analysis: Col Vijay Langer.
Study supervision: Maj Gen M.K Mukherjee, ysm.
Conflicts of interest
All authors have none to declare.
Acknowledgement
The authors wish to thank Mrs. S.R. Patrikar, Lecturer, Dept of Community Medicine, Armed Forces Medical College, Pune, for invaluable support and thorough analysis of the results of the study.
References
- 1.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10:1103–1108. [PubMed] [Google Scholar]
- 2.Sherdian R.L., Tompkins R.G., Burke J.F. Management of burn wounds with prompt excision and immediate closure. J Intensive Care Med. 1994;9:6–7. doi: 10.1177/088506669400900103. [DOI] [PubMed] [Google Scholar]
- 3.Sheridian R.L., Remensnyder J., Prelack K., Petras L., Lydon M. Treatment of the seriously burned infant. J Burn Care Rehabil. 1998;19:115–118. doi: 10.1097/00004630-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Prasanna M., Singh K., Kumar P. Early tangential excision and skin grafting as a routine method of burn wound management: an experience from a developing country. Burns. 1994;20:446–450. doi: 10.1016/0305-4179(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 5.Nada Y., Sasaki K., Nozaki M., Takeuchi M., Chen X., Nakazawa H. The effect of early burn wound excision on regional gastric blood flow in rats. Burns. 1998;24:519–524. doi: 10.1016/s0305-4179(98)00065-5. [DOI] [PubMed] [Google Scholar]
- 6.Salisbury R.E. In: Thermal Burns. McCarthy J.G., editor. vol. I. WB Saunders Company; Philadelphia, PA: 1990. pp. 787–830. (Plastic Surgery). [Google Scholar]
- 7.Miller J.G., Carruthers H.R., Burd D.A. An algorithmic approach to the management of cutaneous burns. Burns. 1992;18:200–211. doi: 10.1016/0305-4179(92)90070-b. [DOI] [PubMed] [Google Scholar]
- 8.Klasen H.J. A review on the non-operative removal of necrotic tissue from burn wounds. Burns. 2000;26:207–222. doi: 10.1016/s0305-4179(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 9.Hummel R.P., Kautz P.D., MacMillan B.G., Altemeier I.N.A. The continuing problem of sepsis-following enzymatic debridement of burns. J Trauma. 1997;14:572–579. doi: 10.1097/00005373-197407000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Silverstein P., Maxwell P.L.D. Enzymatic debridement. In: Boswick J.A., editor. The Art and Science of Burn Care. Aspen Publishers; Rockville: 1987. pp. 75–81. [Google Scholar]
- 11.Krizek T.J., Robson M.C., Groskin M.G. Experimental burn wound sepsis – evaluation of enzymatic debridement. J Surg Res. 1974;17:219–227. doi: 10.1016/0022-4804(74)90112-7. [DOI] [PubMed] [Google Scholar]
- 12.Happ E., Wind S., Kerstein M.D. Pressure ulcers: collaboration in wound care. Is there a reasonable approach? Wounds. 1997;9:34–42. [PubMed] [Google Scholar]
- 13.Sieggreen M., Maklebust J. Debridement: choices and challenges. Adv Wound Care. 1997;10:32–37. [PubMed] [Google Scholar]
- 14.Berger M.M. Enzymatic debriding preparations. Ostomy Wound Manage. 1993;39:61–69. [PubMed] [Google Scholar]
- 15.Fowler E., van Rijswijk L. Using wound debridement to help achieve the goals of care. Ostomy Wound Manage. 1995;41:23S–36S. [PubMed] [Google Scholar]
- 16.Kauzmann W. Factors in interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 17.Miller J.M., Howard F. The interaction of papain, urea, and water-soluble chlorophyll in a proteolytic ointment for infected wounds. Surgery. 1957;43:939–948. [PubMed] [Google Scholar]
- 18.Shi L, Anguiano M, Wright JB, Shroot B: A novel method to assess efficacy of enzymatic wound debriding agents. Abstract. In: Annual Meeting of International Investative Dermatology. Miami Beach, FL; 2003.
- 19.Burke J.F., Golden T.D. A clinical evaluation of enzymatic debridement with papain–urea-chlorophyllin ointment. Am J Surg. 1958;95:828–842. doi: 10.1016/0002-9610(58)90635-4. [DOI] [PubMed] [Google Scholar]
- 20.Cooper G.R., Hodge G.B., Beard J.W. Enzymatic debridement in the local treatment of burns. Am J Dis Child. 1943;65:909–911. [Google Scholar]
- 21.Guzman E.V., Guzman M.G. The enzymatic debridement of suppurations, necrotic lesions and burns with papain. J Int Coll Surg. 1953;20:695–702. [PubMed] [Google Scholar]
- 22.Rosenberg L., Lapid O., Bogdanov-Berezovsky A. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: a preliminary report. Burns. 2004;30:843–850. doi: 10.1016/j.burns.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Garrett T.A. Bacillus subtilis protease: a new topical agent for debridement. Clin Med. 1969;76:11–15. [Google Scholar]
- 24.Accuzyme (Papain, Urea) Ointment US Prescribing Information. Healthpoint, Ltd.; November, 2004. [Google Scholar]
- 25.Gladase (Papain, Urea) Ointment US Prescribing Information. Smith & Nephew Inc.; March, 2005. [Google Scholar]
- 26.Panafil (Papain, Urea, Chlorophylin Copper Complex Sodium) Ointment US Prescribing Information. Healthpoint Ltd.; December, 2004. [Google Scholar]
- 27.Bolton L., Fattu A.J. Topical agents and wound healing. Clin Dermatol. 1994;12:95–120. doi: 10.1016/0738-081x(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 28.Glasser S.R. A new treatment for sloughing wounds: preliminary report. Am J Surg. 1940;40:320–322. [Google Scholar]
- 29.Hebda P.A., Flynn K.J., Dohar J.E. Evaluation of the efficacy of enzymatic debriding agents for removal of necrotic tissue and promotion of healing in porcine skin wounds. Wounds. 1998;10:83–96. [Google Scholar]
- 30.Wright J.B., Lei Shi. Accuzyme papain–urea debriding ointment: a historical review. Wounds. 2003;15:2S–12S. [Google Scholar]


