Abstract
Silver-containing dressings are considered fundamental to the management of infected acute and chronic wounds, specifically burns. The aim of this study was to determine both the spectrum of activity and efficacy of an Alginate/CMC Silver Dressing (ACSP) on planktonic microorganisms by conducting a 21-day repeat-challenge log reduction study. ACSP was found to have a microbiocidal effect, for up to 21 days, on all bacteria and yeast challenged. The results demonstrated an antimicrobial efficacy similar to Hydrofiber Silver Dressing's (HSD)2 up to day 14 for each microorganism tested. However, following a second reinoculation of microorganisms at day 14, ACSP showed antimicrobial efficacy superior to HSD's against a number of opportunistic pathogens, which included Pseudomonas aeruginosa and Candida albicans. The ACSD maintained its antimicrobial action against all microorganisms over the 21-day study period.
Introduction
The high risk of infection in burn patients has justified the need for the use of topical antimicrobials such as silver sulphadiazine. A recent report has demonstrated that the use of silver sulphadiazine should be reconsidered for many wound types.1 Modern silver-impregnated wound dressings are now being used routinely for many wound types, including burns, as antimicrobial barrier dressings.
Many of the wound dressings that are routinely used in burn patients differ significantly in their total silver content and silver ion elution profiles. Consequently, efficacy correlated to ionic silver levels of the dressings will affect barrier property success.2 Wound dressings impregnated with silver are considered fundamental to the management of acute and chronic wounds, specifically burns.3 Maintaining and sustaining levels of ionic silver within the wound–dressing for extended periods may significantly help reduce the need for numerous dressing changes4 and therefore reduce environmental exposure to the healing wound. Most in vitro dressing studies have concentrated solely on short-term use of wound dressings. However, it is important that the correct wound dressings are used and that they remain integral and wearable for extended periods.
If wound dressings are being considered for extended use, longer than the “normal” wear period, a wound dressing needs to be fully evaluated for antimicrobial barrier sustainability and efficacy. Consequently, it is important to determine whether one of the popular silver-impregnated wound dressings composed of ionic silver, CMC and alginates, used routinely in partial thickness burns, have antimicrobial efficacy for up to 21 days.
It was the aim of this in vitro study to determine the antimicrobial efficacy of ACSD (Medline, Mundelein, IL), in comparison with HSD (ConvaTec, Skillman, NJ), in combating a variety of microorganisms frequently found in burn wounds over an extended period of up to 21 days.
Methods
Test Microorganisms
Eight test species of bacteria and yeast were used in this study and included Meticillin- resistant Staphylococcus aureus (ATCC 43300), Meticillin-resistant Staphylococcus epidermidis (ATCC 51625), Vancomycin-resistant Enterococcus faecalis (ATCC 51299), Streptococcus pyogenes (ATCC 19615), Staphylococcus epidermidis (ATCC 12228), Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), and Candida albicans (ATCC 10231).
Before use, all cultured microorganisms were maintained on Protect Preserver Bacterial crybeads and stored at −20 °C. An overnight culture of each test microorganism (except Streptococcus pyogenes) was prepared by placing stock beads into 0.1% Peptone Bacteriological (0.1% wt/vol pH 7.1 ± 0.2; Oxoid, UK) and incubating it at 37 °C ± 1 °C. All cultures were then inoculated into an appropriate medium and maintained at a concentration of 2.0 × 108 cfu mL−1 (0.5 McFarland units) prior to use. An overnight culture of S pyogenes was prepared in Tryptone Soya broth (Oxoid, UK) at 37 °C (± 1 °C). After incubation S pyogenes cells were harvested in Bacteriological Peptone at a concentration of 0.5 MacFarland units.
Test Material
Test and control dressings, measuring 6 × 6 cm, were prepared aseptically with sterile scissors and forceps. All 6 × 6 cm dressing samples were weighed and aseptically cut into smaller pieces, with no piece greater than 1.5 × 1.5 cm, and added to the wound model. The test dressings included ACSD, HSD, and appropriate controls.
ACSD is an alginate and sodium carboxymethylcellulose fibrous dressing incorporating ionic silver. HSD is composed of sodium carboxymethylcellulose fibers and ionic silver.
Inoculation of Samples in the Simulated Wound Model
Into 20-mL volumes of simulated wound fluid, 0.2 mL of each test microorganism was added separately, giving a microbial population of approximately 1.0 × 106 cfu mL−1. Simulated wound fluid was composed of 5.844 0 g sodium chloride, 3.360 4 g sodium hydrogen carbonate, 0.298 2 g potassium chloride, 0.277 5 g calcium chloride, 33.00 g bovine albumin, and 1,000 mL deionized water. Two flasks containing 20 mL of simulated wound fluid were prepared for each product.
All aseptic samples of each wound dressing were separately added to a 20-mL inoculated simulated wound fluid solution and shaken for 5 seconds. Each wound model was then incubated at 37 °C for bacteria and 25 °C for C albicans for the duration of the study. All experiments were performed in duplicate.
Plate Counts
At specific time points each wound dressing sample was initially agitated for at least 5 seconds. A 0.5-mL sample of the fluid was then removed from each wound model and added to an appropriate diluent, RM2, to neutralize any residual ionic silver that remained. RM2 was composed of 5.0 g Peptone Water (Oxoid, UK), 10.0 g Thioglycolic Acid–Sodium Salt (Sigma, UK), 10.0 g Sodium thiosulphate (FSA, UK), 10.0 g Lecithin; Soya Bean (BDH, UK), 150 mL Tween 80 (FSA, UK), and 5,000 mL deionized water. A 10-fold serial dilution series was performed and duplicate pour plates prepared to determine the remaining concentration of microorganisms. The 0.5-mL sample taken for serial dilutions was replaced with 0.5 mL fresh simulated wound fluid so that a constant volume of 20 mL was maintained in the wound model.
For each serial 10-fold dilution, a 1.0-mL bacterial suspension was added to molten tryptone soya agar (Oxoid, UK) by the pore plate method and incubated at 30 °C to 35 °C for up to 5 days. Yeast was grown in Sabouraud Dextrose agar (Oxoid, UK) and incubated at 20 °C to 25 °C for up to 5 days. After incubation the colonies on each plate were counted and the result expressed as cfu per milliliter. All flasks were stored at the appropriate temperature for each organism tested during the 21-day study. Total viable counts were performed for each microorganism and dressing tested every day over the 21-day study period.
At day 7 and day 14, each simulated wound model was reinoculated with 0.2 mL, approximately 1.0 × 108 cfu mL−1, of each test microorganism. This was undertaken to determine sustained antimicrobial efficacy of each test dressing.
Results
ACSD demonstrated a sustained microbiocidal activity against all 8 wound microorganisms tested over the 21-day study period. Poor antimicrobial efficacy of Aquacel Ag after 14 days was also noted when tested against S epidermidis (Figure 1). Poor antimicrobial efficacy of HSD (Figure 2) against planktonic E coli was observed between day 14 and day 17. The efficacy of HSD on C albicans (Figure 3) fluctuated after day 14. A sustained antimicrobial efficacy against one of the key opportunistic wound pathogens, P aeuginosa (Figure 4), was not achieved for HSD after the day-14 point when compared to ACSD.
Figure 1.
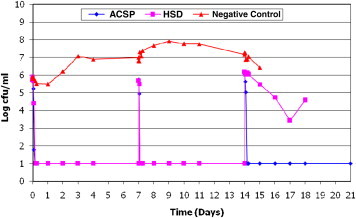
The Antimicrobial Efficacy of ACSD and HSD Against Staphylococcus epidermidis.
Figure 2.
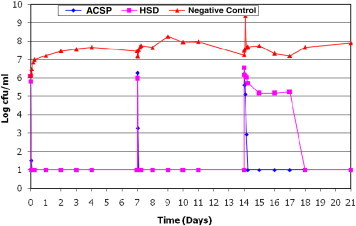
The Antimicrobial Efficacy of ACSD and HSD Against E Coli.
Figure 3.
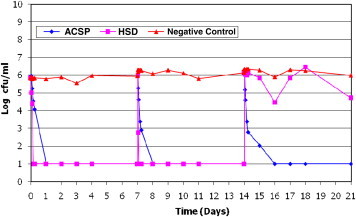
The Antimicrobial Efficacy of ACSD and HSD Against Candida albicans.
Figure 4.
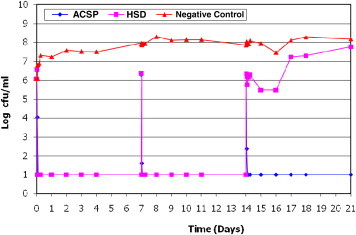
The Antimicrobial Efficacy of ACSD and HSD Against Pseudomonas aeruginosa.
Discussion
All antimicrobial efficacies reported below refer to in essence, the antimicrobial barrier properties of the products tested.
Acute and chronic wounds are documented to be polymicrobial in nature.5-10 It is therefore important to demonstrate that wound dressings, and in particular those containing silver, have antimicrobial barrier efficacy on a wide range of microorganisms known to inhabit a wound bed.11 Bacteria and yeast that have been documented to constitute a concern in wounds include the opportunistic pathogens P aeruginosa,12,13S aureus,14,15 and S pyogenes.16 Ionic silver has been documented to have a significant role to play in reducing the microbial bioburden of burn and chronic wounds that are infected or at risk of infection.4 Consequently, within this study the antimicrobial barrier efficacy of ACSD was investigated and compared with that of HSD over a 21-day test period. Both ACSD and HSD exhibited sustained antimicrobial activity within the dressing up to 14 days' exposure to bacteria and yeast. However, ACSD demonstrated an enhanced efficacy when compared to HSD on 3 bacteria and 1 yeast tested after 14 days. The sustained activity of ACSD within the dressing was established despite reinoculation of each test organism. Poor efficacy of HSD following reinoculation was noted against S epidermidis, C albicans, P aeruginosa, and E coli. The implications and importance clinically of a dressing's ability to sustain its activity over a 7-day period and beyond is an area of great debate.
The results in this study suggest that the ability of ionic silver within the dressing, made available to kill microorganisms, are sustained and maintained for longer time periods by ACSD than by HSD. This is important when extended wear time of a wound dressing is needed. Clinically this is significant for the patient and also has cost implications.
After 14 days the antimicrobial efficacy of HSD diminished significantly. However, ACSD continued to kill all the test organisms for up to 21 days. Despite this it is wise to be cautious when extrapolating in vitro results to the clinical situation.
In conclusion ACSD maintains a sustained level of antimicrobial activity within the dressing for up to 21 days and, with its inherent absorbency capabilities and tensile strength, may reduce dressing change frequency.
Footnotes
Conflict of interest: The authors are all employees of Advanced Medical Solutions Ltd.
1ACSD is Maxorb Extra Ag, a registered trademark of Medicine Industries, Inc.
2HSD is Aquacel, registered trademark of ConvaTec, Ltd.
HSD is Aqualcel, a registered trademark of Conva Tec, Ltd.
References
- 1.Wasiak J., Cleland H., Campbell F. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 2008;8(4) doi: 10.1002/14651858.CD002106.pub3. CD002106. [DOI] [PubMed] [Google Scholar]
- 2.Lansdown A.B.G. A review of silver in woundcare: facts and fallacies. Br J Nurs. 2004;13:6–19. doi: 10.12968/bjon.2004.13.Sup1.12535. [DOI] [PubMed] [Google Scholar]
- 3.Lansdown A.B.G. Silver 1: its antimicrobial properties and mechanism of action. J Wound Care. 2002;11:125–131. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 4.Lo S.F., Chang C.J., Hu W.Y., Hayter M., Chang Y.T. The effectiveness of silver-releasing dressings in the management of non-healing chronic wounds: a meta-analysis. J Clin Nurs. 2009;18(5):716–728. doi: 10.1111/j.1365-2702.2008.02534.x. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence J.C. The bacteriology of burns. J Hosp Infect. 1985;6(B):3–17. doi: 10.1016/s0195-6701(85)80081-5. [DOI] [PubMed] [Google Scholar]
- 6.Hill K.E., Davies C.E., Wilson M.J., Stephens P., Harding K.G., Thomas D.W. Molecular analysis of the microflora in chronic venous leg ulceration. J Med Microbiol. 2003;52(Pt 4):365–369. doi: 10.1099/jmm.0.05030-0. [DOI] [PubMed] [Google Scholar]
- 7.Davies C.E., Wilson M.J., Hill K.E. Use of molecular techniques to study microbial diversity in the skin: chronic wounds re-evaluated. Wound Repair Regen. 2001;9:332–340. doi: 10.1046/j.1524-475x.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 8.Percival SL, Bowler PG: Understanding the effects of bacterial communities and biofilms on wound healing. World Wide Wounds. 2004. July. Available at www.worldwidewounds.com. Accessed July 2009.
- 9.Andersen A., Hill K.E., Stephens P., Thomas D.W., Jorgensen B., Krogfelt K.A. Bacterial profiling using skin grafting, standard culture and molecular bacteriological methods. J Wound Care. 2007;16(4):171–175. doi: 10.12968/jowc.2007.16.4.27025. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane C.A., Freeman K., Woods E., Welsby S., Percival S.L. Biofilm evidence and the microbial diversity of horse wounds. Can J Microbiol. 2009;55(2):197–202. doi: 10.1139/w08-115. [DOI] [PubMed] [Google Scholar]
- 11.Leaper D.J. Silver dressings: their role in wound management. Int Wound J. 2006;3(4):282–294. doi: 10.1111/j.1742-481X.2006.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang C.I., Kim S.H., Park W.B. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guggenheim M., Zbinden R., Handschin A.E., Gohritz A., Altintas M.A., Giovanoli P. Changes in bacterial isolates from burn wounds and their antibiograms: a 20- year study (1986-2005) Burns. 2009;35(4):553–560. doi: 10.1016/j.burns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Gardner S.E., Frantz R.A., Saltzman C.L., Dodgson K.J. Staphylococcus aureus is associated with high microbial load in chronic wounds. Wounds. 2004;16:251–257. [Google Scholar]
- 15.Aufiero B., Duanmu Z., Guo M., Meduri N.B., Murakawa G.J., Falkow S. Staphylococcus aureus infection of human primary keratinocytes. J Dermatol Sci. 2004;36(3):173–175. doi: 10.1016/j.jdermsci.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Wright A.E., Fleming A., Colebrook L. The conditions under which the sterilisation of wounds by physiological agency can be obtained. Lancet. 1918;i:831–838. [Google Scholar]


