Abstract
Background
Haematological abnormalities are among the most common complications of HIV. These involve all lineages of blood cells. Bone marrow studies form integral part of complete workup of the HIV positive patients specially when they present as case of pyrexia of unknown origin (PUO), refractory anaemia and pancytopenia.
Method
55 HIV infected symptomatic patient requiring bone marrow examination were included in the study. Relevant clinical history, baseline haematological investigations including full blood count, CD4 cell counts using flow cytometry were recorded.
Results
Median ANC values in males were found to be significantly lower than females (p = 0.046). CD4 cell count statistically significantly correlated with age, TLC, ANC & platelet count. Anaemia was present in 45 patients and out of which 66.66% patients had normocytic normochromic anaemia. Iron deficiency anaemia was present in (37.77%) patients and anaemia of chronic disease in (62.22%) patients. 2 patients had anaemia of the critically ill.
Two patients had non-Hodgkin's lymphoma (NHL) and showed lymphoma deposit in the bone marrow. Gelatinous degeneration was seen in 3 patients. Ill formed epithelioid cell granulomas were seen in 7 cases, and 2 cases were positive for acid fast bacilli (AFB). Haemophagocytosis was seen in 8 cases; two cases later diagnosed as a case of infection induced HLH. Leishmania donovani (LD) bodies seen in 2 cases.
Conclusions
Bone marrow study is an important investigation in HIV infected symptomatic patients with peripheral haematological abnormalities.
Keywords: CD4 cell count, Bone marrow examination, HIV infection, Anaemia of critically ill, Haemophagocytic lymphohistiocytosis
Introduction
Haematological abnormalities are among the most common complications of human immune deficiency virus (HIV) infection. These involve all the lineages of blood cells.1 The mechanisms of these changes are multiple. It causes both quantitative and qualitative marrow defects. Immune cytopenias can occur directly due to HIV infection, whereas the effects of opportunistic infections, lymphomas, malignancy and anti-retroviral therapy (ART) also play an important role. Bone marrow abnormalities are common in HIV infection and increase in frequency with advancing disease.1 The consequences of these haematologic problems are twofold. First, they are associated with morbidity in themselves that can adversely alter the patient's quality of life such as from anaemia (fatigue and dyspnoea), leucopenia (infections) and thrombocytopenia (bleeding). Second, they hinder treatment of the primary viral infection, secondary infections and neoplastic complications.
In our scenario, every microcytic hypochromic anaemia is considered as iron deficiency anaemia. But now we know that it can be anaemia of chronic disease or anaemia of critically ill. Their differentiation from iron deficiency anaemia is of utmost importance because of difference in treatment.2
Bone marrow studies form integral part of complete workup of the HIV positive patients especially when they present as a case of pyrexia of unknown origin (PUO), refractory anaemia and pancytopenia. A number of characteristic but nonspecific, morphologic abnormalities of the bone marrow of AIDS patients have been reported.3
Haemophagocytic lymphohistiocytosis (HLH) is a hyper inflammatory syndrome characterised by fever, hepatosplenomegaly, cytopenias and evidence of lymphophagocytosis by lymphocytes along with and other characteristic lab abnormalities.4 HLH can be primary (familial) or secondary (acquired). Secondary (acquired) HLH has been associated with a variety of viral, bacterial, fungal, and parasitic infections, as well as collagen-vascular diseases.5–8 Few case reports of HIV infected individuals also developing this dreadful and fatal condition has been published.9 Extra lymphatic presentation of non-Hodgkin's lymphomas occurs in up to 90% of patients with HIV infection and lymphoma has been reported to involve the bone marrow in 50% of cases.10
The present study is aimed to assess the various haematological and bone marrow abnormalities seen in symptomatic HIV patients requiring bone marrow studies with special mention of functional iron deficiency, anaemia of critically ill and HLH.
Materials and methods
A cross sectional study was carried out in Dept of Pathology, at a tertiary health care centre between June 2009 and June 2012. All the HIV positive symptomatic patients requiring bone marrow examination were included. Bone marrow aspirate and biopsy was performed as a part of investigation of pyrexia of unknown origin (PUO), unresolving hepatosplenomegaly, anaemia, leucopenia, neutropenia, lymphopenia and thrombocytopenia. Patients who were on antiretroviral therapy were excluded. This was done to reduce the confounding effect of antiretroviral drug induced bone marrow suppression. Diagnosis of HIV was made in these patients by ELISA and Western Blot. A total of 55 patients were studied. Detailed clinical history was obtained. The following haematological investigations were carried out for all patients: haemoglobin, total leucocyte count (TLC), differential leucocyte count (DLC), erythrocyte sedimentation rate (ESR), platelet count, mean corpuscular volume (MCV), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular haemoglobin (MCH), packed cell volume (PCV), reticulocyte count, peripheral smear for blood picture. Absolute lymphocyte count was calculated as absolute lymphocyte count (ALC) (cells/μL) = TLC × DLC (%). Serum iron studies were carried out in all the anaemic patients. CD4 count was carried using flow cytometry in all the patients.
We studied bone marrow for cellularity, dysplasia, plasma cell numbers, dysplastic changes, fibrosis, bone marrow iron status with special mention of diagnosis of anaemia of critically ill (functional iron deficiency), granulomas, evidence of haemophagocytosis, opportunistic infections and other pathognomonic features of systemic infections or malignancies. Bone marrow aspiration smears were stained using Leishman's stain, whereas trephine biopsy was stained with haematoxylin and eosin stain. Iron stores in bone marrow aspirates were assessed in 50 patients and grading of 0–6 was done as per Gale et al.11 In remaining 5 cases where bone marrow aspirate were suboptimal, assessment of iron status was done on the bone marrow biopsy. Patients were categorised into Cat 1 = grade 0 & 1 (absence & diminished iron store), Cat 2: grade ≥2 (normal to increased iron stores). For assessment of bone marrow fibrosis, reticulin stain was done on 5 u thick sections and semi quantitative grading (0–3) was done as per Bauermeister's/WHO grading (2008).12 Bone marrow plasma cells were assessed in the percentage on bone marrow aspirates and bone marrow biopsies. Ziehl–Neelsen (ZN) staining for Acid fast bacilli (AFB) was done in selected cases. Special stains for fungus and immunohistochemistry was done on case to case basis. All the bone marrow aspiration and biopsy specimens were examined and interpreted by the same pathologist, to avoid inter-observer variation.
Statistical analysis
Statistical analysis was done using SPSS version 17. Mean and median values were compared for various quantitative variables. Non-parametric tests were used wherever medians were compared. A p value <0.05 was taken as statistically significant.
Results
The mean age ± SD of 55 HIV infected individual was 41.15 ± 9.96. About two third of individuals, 69% (n = 38) were males and 31% (n = 17) were females. On initial presentation, patients presented with fever (54.5%; 30), generalised weakness (36.3%; 20), diarrhoea (9.1%; 5), loss of weight/appetite (9.1%; 5), and others (12.7%; 7) with non-specific symptoms. Out of 55 patients, 35 patients had more than one symptom. Various haematological parameters were evaluated in 55 HIV infected individuals who underwent bone marrow studies and results are summarised in Table 1.
Table 1.
Evaluation of all the haematological parameters (N = 55).
| Parameters | Range | Mean/median |
|---|---|---|
| Hb gm %a | 4–14 | 9.38a |
| TLC (cells/μL) | 850–12,100 | 4100 |
| ANC (cells/μL) | 450–7840 | 2640 |
| ALC (cells/μL) | 161–3751 | 957 |
| Neutrophils (%) | 33–83 | 60 |
| Lymphocytes (%) | 11–56 | 24 |
| Platelets (cells/μL) | 18,000–476,000 | 186,000 |
| Reticulocyte count (corrected) % | 0.6–1.6 | 1.2 |
| CD4 (cells/μL) | 50–760 | 390 |
Mean & SD was calculated for Hb estimation.
While comparing these parameters between male and female patients, we found the distribution skewed in most of the parameters except Hb. Thus we used unpaired ‘t ’ test while comparing mean values of Hb and for other parameters ‘Mann–Whitney U’ test was used for comparing medians. On doing this analysis, median ANC values in males were found to be significantly lower than females (p = 0.046). The other variables like Hb, TLC, ALC, polymorphs, lymphocytes, platelets and CD4 count did not show any significant difference. CD4 cell count statistically significantly correlated with age, TLC, ANC & platelet count (Table 2). In order to find if there is any association between platelet counts and CD4 counts. We divided patients into two categories based on CD4 count. Cat 1 = CD4 ≤ 200, Cat 2 = CD4 > 200. Median platelet counts in the category of patients with CD4 ≤ 200 and ≤400 was lesser than that of CD4 > 200 and >400 respectively, but the difference was not statistically significant. (p: 0.56 and 0.73 respectively by using Mann–Whitney U test.) Fig. 1 shows the regression analysis scatter plot, where platelet count correlates with CD 4 cell count.
Table 2.
Correlation analysis between CD4 and various quantitative parameters.
| Parameter | Mean (SD)/median | Correlation coefficient | p Value |
|---|---|---|---|
| Age | 41.15 ± 9.96a | −0.419a | 0.001 |
| Hb% | 9.38 ± 2.17a | 0.043a | 0.75 |
| TLC | 4100 | 0.293 | 0.030 |
| ANC | 2640 | 0.267 | 0.049 |
| ALC | 957 | 0.238 | 0.080 |
| Polymorphs | 60 | 0.022 | 0.871 |
| Lymphocytes | 24 | −0.024 | 0.862 |
| Platelet count | 186,000 | 0.274 | 0.041 |
Pearson's coefficient was used for age and Hb %; Spearman's for other variables.
Fig. 1.
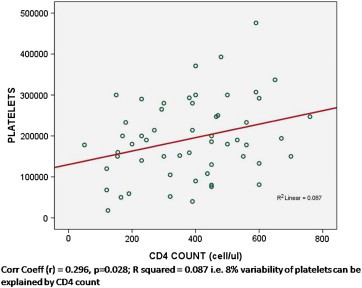
Scatter plot showing relationship between platelet count and CD4 cell count.
Anaemia was present in 45 (81.8%) patients, red cell indices and peripheral blood smears were evaluated for morphological classification. 30 (66.66%) patients had normocytic normochromic anaemia, microcytic hypochromic in 10 (22.22%) and macrocytic in 5 (11.11%) patients. Leucopenia was observed in 7 (12.72%). Thrombocytopenia was present in 11 (20%) and 10 (18.18%) patients had pancytopenia. Out of 55 cases, bone marrow aspiration was reported in 50 cases. Three cases had dry tap and 2 were grossly haemodiluted and suboptimal for reporting. In these cases bone marrow biopsy was relied upon for assessment of all the parameters. All the bone marrow changes are summarised in Table 3.
Table 3.
Summary of all the bone marrow changes based on bone marrow aspirate and bone marrow biopsy findings.
| Bone marrow change | No. of cases (N = 55) |
|---|---|
| Normocellular marrow | 37 |
| Megaloblastic changes | 13 |
| Dyserythropoiesis | 2 |
| Dysgranulopoiesis | 5 |
| Dysmegakaryopoiesis | 1 |
| Increased plasma cells >5% | 11 |
| Haemophagocytosis | 8 |
| Epithelioid cell granulomas | 7 |
| AFB positive cases | 3 |
| LD bodies | 2 |
| Gelatinous degeneration | 3 |
| Lymphoma deposits in bone marrow | 2 |
Normocellular marrow was present in 37 (67.3%) patients, 10 (18.2%) showed hypercellular and 8 (14.5%) hypocellular marrow. Myelodysplasia was present in the 8 cases (14.5%), most commonly in granulocytic series, 5 cases (9.1%), followed by erythroid series, 2 cases (3.6%) and megakaryocytic series showed dysplasia in 1 (1.8%) case. Trilineage dysplasia was seen in one case. Total of 15 (27.27%) patient showed absent to reduced iron stores, 12 (21.8%) normal and 23 (41.8%) showed increased iron stores. On the basis of red cell indices, serum iron studies and bone marrow iron status, in our study only 17/45 (37.77%) patients had iron deficiency anaemia and 28/45 (62.22%) patient had anaemia of chronic disease. We diagnosed 2 of the patients as case of anaemia of the critically ill, both had septicaemia and were treated in the intensive care unit (ICU).
We compared bone marrow iron grading with mean Hb values and with CD4 cell count. Unpaired ‘t’ test was used to compare Hb values and ‘Mann–Whitney U’ test for comparing median CD4 cell counts with grading of iron stores. In our study we found, no statistically significant difference between mean Hb levels and median CD4 counts with category 1 and category 2 of bone marrow iron stores (p > 0.05). 24 (43.6%) patients had plasma cells <2%, 15 patients (27.3%) had between 2 and 5% and 11 (20%) had >5% plasma cells (Fig. 2A).
Fig. 2.
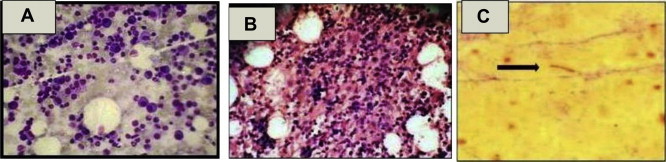
(A) Photomicrograph of bone marrow aspirate (Leishman's stain: 400×) showing increased plasma cells. (B) Photomicrograph of bone marrow biopsy (haematoxylin and eosin stain: 400×) shows an ill formed epithelioid cell granuloma. (C) Photomicrograph of bone marrow aspirate (ZN stain: 1000×) showing acid fast bacillus.
Megaloblastic changes were seen in 13/55 (23.6%) cases (based on bone marrow aspirate and bone marrow aspirate findings) of which 8 cases showed reduced serum vitamin B12 levels. Haemophagocytosis was seen in 8 cases; however significant haemophagocytosis was present in only 3 cases (>3%),13 one of them had associated fever, splenomegaly, cytopenias, raised ferritin and hypertriglyceridemia. He was later diagnosed as a case of infection induced HLH as per diagnostic criteria for HLH-200413 (Fig. 3A–D).
Fig. 3.
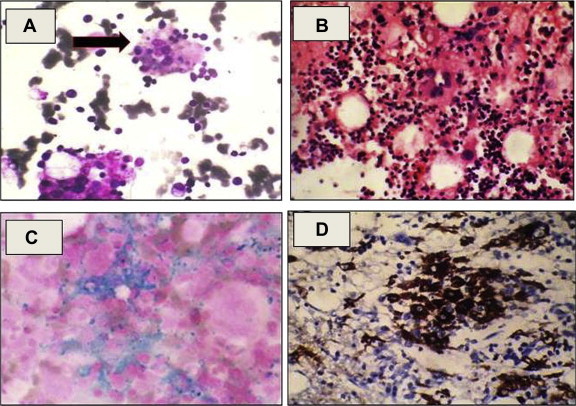
(A) Photomicrograph of bone marrow aspirate (Leishman's stain: 400×) showing histiocytes with evidence of haemophagocytosis. (B) Photomicrograph of bone marrow biopsy (haematoxylin and eosin stain: 400×) showing focal collections of histiocytes. (C) Photomicrograph of bone marrow aspirate (Perl stain: 400×) showing histiocytes stuffed with iron. (D) IHC photomicrograph of bone marrow biopsy (400×) showing strong cytoplasmic positivity of CD68 in the histiocytes.
Ill formed epithelioid cell granulomas were seen in 7 cases, all of them admitted as case of PUO and 2 showed positivity for acid fast bacilli (AFB) (Fig. 2B, C). Interestingly we had detected Leishmania donovani (LD) bodies in 2 cases who presented as case of pyrexia of unknown origin (PUO) and had massive splenomegaly. Two patients had non-Hodgkin's lymphoma (NHL), diffuse large B cell lymphoma (DLBCL) plasmablastic variant and showed lymphoma deposit in the bone marrow. Gelatinous degeneration was seen in three patients, when they were further investigated one of the patient found to have paravertebral DLBCL (Fig. 4A–C).
Fig. 4.
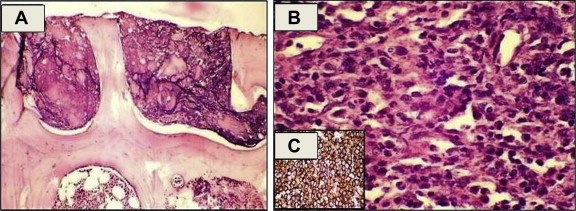
(A) Photomicrograph of bone marrow biopsy (haematoxylin and eosin stain: 100×) showing gelatinous degeneration. (B) Photomicrograph of biopsy from paravertebral mass (haematoxylin and eosin stain: 400×) showing diffuse large cell lymphoma. (C) IHC photomicrograph of bone marrow biopsy (400×) diffuse sheets of atypical lymphoid cells showing strong positivity for CD 20.
Discussion
Haematological complications are a common cause of mortality in HIV infected patients. In present study various haematological abnormalities were observed in isolation or in combination. Anaemia was present in 81.8% patients. In our country where iron deficiency anaemia is most common cause of anaemia, we tend to miss or do not even consider the possibilities of other causes. The dynamics of the transport, distribution, and recycling of iron are tightly regulated within humans. While much remains to be discovered regarding the control of iron balance, hepcidin, a recently discovered 25 amino-acid protein, is believed to be critical to this process. Hepcidin serves as a negative regulator, works as a movable fence and when elevated, results in reduced intestinal iron absorption and macrophage iron release. Erythroblasts do not get iron from the macrophages despite sufficient iron stores (functional iron deficiency).14
Hepcidin often increases in response to inflammation, a process that is thought to be responsible for much of the iron abnormalities that are a hallmark of anaemia of chronic disease. On the basis of red cell indices, serum iron studies and bone marrow iron status, in our study only 37.77% patients had iron deficiency anaemia and 62.22% patient had anaemia of chronic disease. Our results are slightly different from those of Dikshit et al in which they found iron deficiency anaemia in 49.2% cases and anaemia of chronic disease in 50.8% of HIV infected cases (Fig. 4).15
Anaemia of the critically ill also known as anaemia of intensive care unit (ICU) patients resembles anaemia of chronic disease, but it is an acute form of anaemia of inflammatory disease and characterised by blunted erythropoietic response. Approximately 90% of the ICU patients have low serum iron concentration and low total iron binding capacity, with normal or elevated serum ferritin concentration. Serum erythropoietin (EPO) levels are blunted with only mild elevation which results in minor reticulocyte response, and occurs due to pro inflammatory mediators like interleukin 1-β, interleukin-6, tumour necrosis factor-α (TNF-α).16 Two of our cases had anaemia of the critically ill. Normocytic normochromic anaemia was the most common haematological abnormality, occurring in 66.66% patients, this is in agreement with another Indian study.17 Cytopenias often cause symptoms and contribute to the complications suffered by patients with acquired immunodeficiency syndrome (AIDS) like infections, anaemia and bleeding. These are more common in women. Prevalence of thrombocytopenia is reported to be higher among persons with AIDS, older persons, homosexuals and intravenous drug users.18 As per Fauci et al19 in HIV patients if CD4 <400, 10% patients will have platelet count <150,000 whereas for CD4 >400, it is only 3%; but in our study we found that for CD4 <400, 20% of the patient had platelet count <150,000 whereas for CD4 >400, it was 16.36%. On correlation analysis of various parameters using Pearson's and Spearman's correlation analysis we found significant correlation between CD4 count, age, TLC, ANC & platelet count.
In our study, inadequate aspiration of the bone marrow was seen in 3 cases. It was probably due to the focal fibrosis (>grade 1), which was seen in 25% of cases. This finding is similar to that of Karcher et al who had found marrow fibrosis in 20% cases.20 However, in one Indian study observed marrow fibrosis in 54% of HIV infected cases.21 In our study, majority (67.3%) of the patients showed normocellular marrow, results are similar to other studies.22
In our study dysplastic changes were seen most commonly in the granulocytic series which is in agreement with study by Sitalakshmi et al.21 Other abnormalities which are seen in the bone marrow matrix of HIV infected cases are serous atrophy or gelatinous degeneration.19 Other causes of serous atrophy are post chemotherapy, malnutrition and anorexia nervosa. Plasma cells are often increased in the marrow of HIV-infected patients seen in 31–85% of patients.19,21 These may represent a physiological response to antigenic stimulation by viruses or other infective agents, or may be secondary to dysregulated B cell proliferation due to HIV itself. We also observed increased plasma cells in the bone marrow, between 2–5% in 27.3% patients and >5% in 20% of cases.
Opportunistic infections are common in HIV infection and bone marrow involvement is commonly seen with Mycobacterium avium complex, Mycobacterium tuberculosis, Histoplasma, Cryptococcus, Toxoplasma, Cytomegalovirus, Leishmania, Pneumocystis carinii and Parvovirus B 19. Ill formed epithelioid cell granulomas were seen in 7 cases who presented as a case of PUO. These findings are consistent with Castella et al23 and Calore et al24 they had reported granulomas in 16% and 12% among the HIV infected cases respectively.
HIV infection is associated with T-cell depletion and cytokine dysregulation (low T-cell levels of interferon gamma, interleukin IL-2, and IL-12). This leads to diminished activation and intracellular killing of Leishmania by macrophages. Consequently, Leishmania infection accelerates the course of HIV infection and is associated with poorer prognosis and a higher mortality.25,26 Leishmania enhances HIV viral propagation especially by modulation of cytokine (tumour necrosis factor α and IL-1) secretion by infected macrophages.27,28 We also saw LD bodies in two cases.
HLH is a distinct clinical entity characterised by fever, pancytopenia, splenomegaly, and hemophagocytosis in bone marrow, liver, or lymph nodes. Histiocytic phagocytosis of erythroid cells and occasionally granulocytes and platelet has been described in HIV cases,9 but this is a nonspecific finding that may also occur in a variety of viral, fungal and bacterial infections. As a result of HIV infection, the marrow produces a histiocytic reaction, which varies from increased number of histiocytes to a full blown hemophagocytic syndrome with severe pancytopenia.29 Extra lymphatic presentation of non-Hodgkin's lymphomas occurs in up to 90% of patients with HIV infection and lymphoma has been reported to involve the bone marrow in 50% of cases.10 In our study 02 of the patients were known case of non-Hodgkin's lymphoma (NHL) and showed lymphoma deposits in the bone marrow.
Conclusion
With the continuing rise in prevalence of HIV infection worldwide, it is important for the pathologist to recognise the haematological abnormalities and morphological changes in the bone marrow associated with HIV infection. The aetiology of these findings are possibly either due to direct effects of HIV, nutritional deficiencies, opportunistic infections of marrow or the use of marrow suppressive agents. Further research on haematological complications of HIV disease will lead to effective management of cases and reduce the morbidity and mortality from this dreaded disease. There are several limitations with our study. First our sample size was small and second we have not divided the patient into AIDS and non-AIDS group but all our patient were symptomatic HIV positive individuals who required bone marrow examination. We have only studied patients who had not been previously treated with ART to avoid confounding factors in form of changes caused by ART itself.
In conclusion, peripheral and bone marrow abnormalities are common in HIV infected individuals. Functional iron deficiency is very important cause of anaemia in HIV positive patients. These abnormalities become more frequent as the disease progresses. Bone marrow study is an important investigation in the management of HIV infected patients with peripheral haematological abnormalities. It is a relatively safe and low cost procedure that can contribute to a comprehensive evaluation of cytopenias which lead to various complications.
Intellectual contribution of authors
Study concept: Col Jyoti Kotwal, Maj Vikram Singh.
Drafting & manuscript: Col Jyoti Kotwal, Maj Vikram Singh, Anupam Kotwal, Brig Vibha Dutta, sm, Maj Gen Velu Nair, avsm, vsm∗∗.
Statistical analysis: Col Jyoti Kotwal, Anupam Kotwal, Maj Vikram Singh.
Study Supervision: Col Jyoti Kotwal, Brig Vibha Dutta, sm, Maj Gen Velu Nair, avsm, vsm∗∗.
Conflicts of interest
All authors have none to declare.
References
- 1.Kirchhoff F., Silvestri G. Is Nef the elusive cause of HIV-associated hematopoietic dysfunction? J Clin Invest. 2008;118:1622–1625. doi: 10.1172/JCI35487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Bellis R.J. Anaemia in critically ill patients: incidence, etiology, impact, management and use of treatment guidelines and protocols. Am J Health Syst Pharm. 2007;64:14–21. doi: 10.2146/ajhp060602. [DOI] [PubMed] [Google Scholar]
- 3.Frontiera M., Myers A.M. Peripheral blood and bone marrow abnormalities in the acquired immunodeficiency syndrome. West J Med. 1987;147:157–160. [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S., Weitzman S. Primary and secondary haemophagocytic lymphohistiocytosis: clinical features, pathogenesis and therapy. Expert Rev Clin Immunol. 2010 Jan;6(1):137–154. doi: 10.1586/eci.09.58. [DOI] [PubMed] [Google Scholar]
- 5.Onishi R., Namiuchi S. Hemophagocytic syndrome in a patient with rheumatoid arthritis. Intern Med. 1994;33:607–611. doi: 10.2169/internalmedicine.33.607. [DOI] [PubMed] [Google Scholar]
- 6.Wong K.F., Hui P.K., Chan J.K., Chan Y.W., Ha S.Y. The acute lupus hemophagocytic syndrome. Ann Intern Med. 1991;114:387–390. doi: 10.7326/0003-4819-114-5-387. [DOI] [PubMed] [Google Scholar]
- 7.Kumakura S., Ishikura H., Munemasa S., Adachi T., Murakawa Y., Kobayashi S. Adult onset Still's disease associated hemophagocytosis. J Rheumatol. 1997;24:1645–1648. [PubMed] [Google Scholar]
- 8.Morris J.A., Adamson A.R., Holt P.J., Davson J. Still's disease and the virus-associated haemophagocytic syndrome. Ann Rheum Dis. 1985;44:349–353. doi: 10.1136/ard.44.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T.L., Wong W.W., Chiou T.J. Hematophagocytic syndrome: an unusual manifestation of acute human immunodeficiency virus infection. Int J Hematol. 2003;78:450–452. doi: 10.1007/BF02983819. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler J.L., Beckstead J.A., Volberding P.A. Non-Hodgkin's lymphoma in 90 homosexual men: relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:565–570. doi: 10.1056/NEJM198408303110904. [DOI] [PubMed] [Google Scholar]
- 11.Gale E., Torrance J., Bothwell T. The quantitative estimation of total iron stores in human bone marrow. J Clin Invest. 1963;42:1076–1078. doi: 10.1172/JCI104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. [PubMed] [Google Scholar]
- 13.Henter J.I., Horne A., Arico M. HLH-2004: diagnostic and therapeutic guidelines for haemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 14.Roy C.N., Andrews N.C. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Dikshit Byomakesh, Wanchu Ajay, Sachdeva Ravinder K., Sharma Aman, Das Reena. Profile of hematological abnormalities of Indian HIV infected individuals. BMC Blood Disord. 2009;9:5. doi: 10.1186/1471-2326-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stubbs J.R. Alternatives to blood product transfusion in the critically ill: erythropoietin. Crit Care Med. 2006;34:160–169. doi: 10.1097/01.CCM.0000214290.11479.5C. [DOI] [PubMed] [Google Scholar]
- 17.Patwardhan M.S., Golwilkar A.S., Abhyankar J.R., Atre M.C. Hematological profile of HIV positive patients. Indian J Pathol Microbiol. 2002;45:147–150. [PubMed] [Google Scholar]
- 18.Kouri Y.H., Borkowsky W., Nardi M., Karpatkin S., Basch R.S. Human megakaryocytes have a CD4 molecule capable of binding human immunodeficiency virus-1. Blood. 1993;81:2664–2670. [PubMed] [Google Scholar]
- 19.Fauci Anthony S., Clifford Lane H. Human immunodeficiency virus disease: AIDS and related disorders. In: Longo Dan L., Kasper Dennis L., Larry Jamesson J., Fauci Anthony S., Hauser Stephen L., Loscalzo Joseph., editors. Harrison's Principles of Internal Medicine. 18th ed. McGraw Hills; New York: 2012. pp. 1506–1587. [Google Scholar]
- 20.Karcher D.S., Frost A.R. The bone marrow in human immunodeficiency virus-related disease morphology and clinical correlation. Am J Clin Pathol. 1991;95:63–71. doi: 10.1093/ajcp/95.1.63. [DOI] [PubMed] [Google Scholar]
- 21.Sitalakshmi S., Srikrishna A., Damodar P. Hematological changes in HIV infection. Indian J Pathol Microbiol. 2003;46:180–183. [PubMed] [Google Scholar]
- 22.Sun N.C.J., Shapshak P., Lachant N.A. Bone marrow examination in patients with AIDS and AIDS-related complex (ARC) Am J Clin Pathol. 1989;92:589–594. doi: 10.1093/ajcp/92.5.589. [DOI] [PubMed] [Google Scholar]
- 23.Castella A., Croxson T.S., Mildvan D., Witt D.H., Zalusky R. The bone marrow in AIDS. A histologic, hematologic, and microbiologic study. Am J Clin Pathol. 1985;84:425–432. doi: 10.1093/ajcp/84.4.425. [DOI] [PubMed] [Google Scholar]
- 24.Calore E.E., Tanaka P.Y., Perez N.M., Almeida L.V. Bone marrow pathology in AIDS. Pathol Res Pract. 2004;200:591–597. doi: 10.1016/j.prp.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Pintado V., Martin-Rabadan P., Rivera M.L. Visceral leishmaniasis in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients. A comparative study. Medicine. 2001;80:54–73. doi: 10.1097/00005792-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C., Papadopoulou B., Tremblay M.J. Leishmania infantum enhances human immunodeficiency virus type-1 replication in primary human macrophages through a complex cytokine network. Clin Immunol. 2004;113:81–88. doi: 10.1016/j.clim.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Tremblay M., Olivier M., Bernier R. Leishmania and the pathogenesis of HIV infection. Parasitol Today. 1996;12:257. doi: 10.1016/0169-4758(96)10021-1. [DOI] [PubMed] [Google Scholar]
- 28.Bernier R., Turco S.J., Olivier M. Activation of human immunodeficiency virus type 1 in monocytoid cells by the protozoan parasite Leishmania donovani. J Virol. 1995;69:7282–7285. doi: 10.1128/jvi.69.11.7282-7285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grateau G., Bachmeyer C., Blanche P. Hemophagocytic syndrome in patients infected with the human immunodeficiency virus: nine cases and a review. J Infect. 1997;34:219–225. doi: 10.1016/s0163-4453(97)94227-4. [DOI] [PubMed] [Google Scholar]


