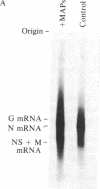
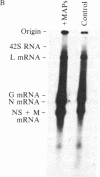
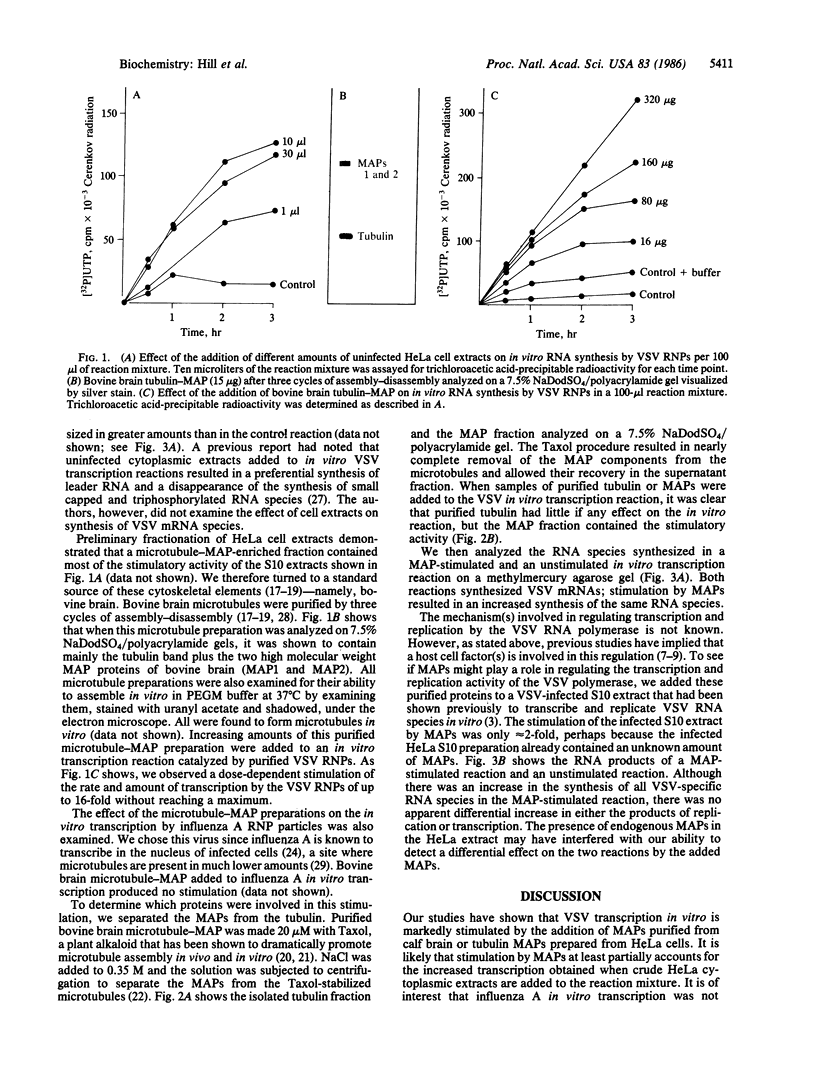
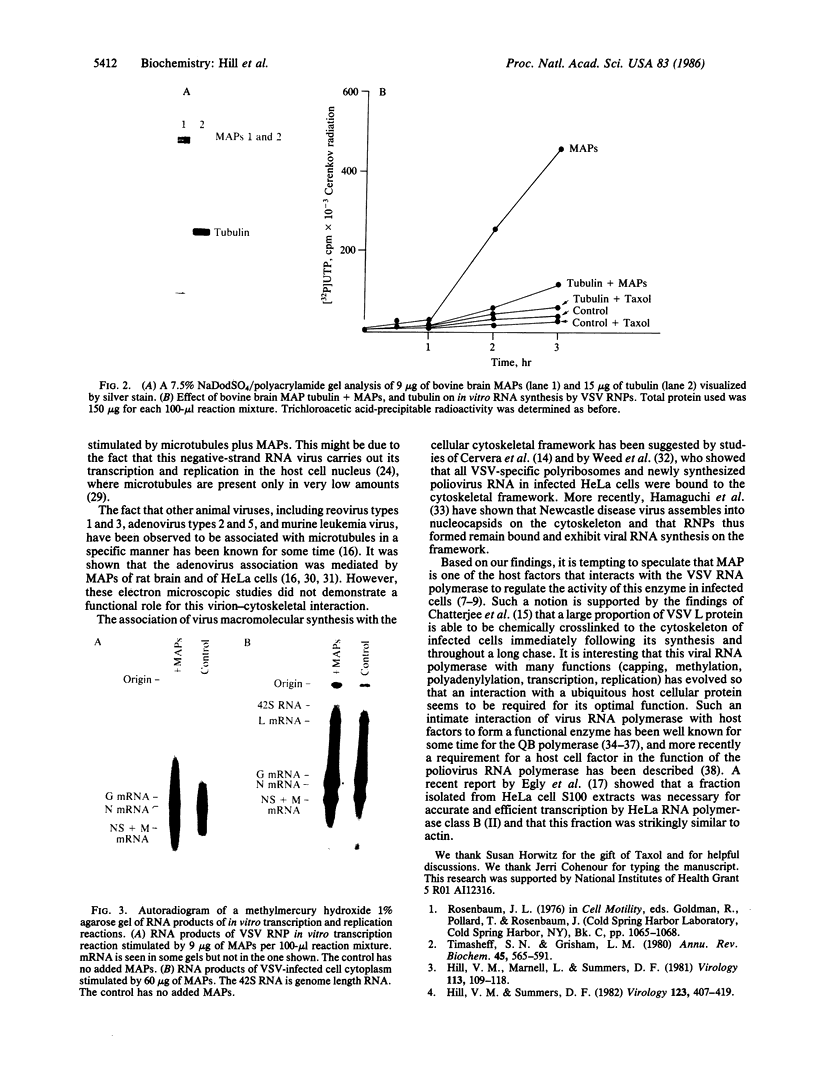
Abstract
Microtubule-associated proteins purified from bovine brains stimulated the in vitro transcription and replication reactions of vesicular stomatitis virus. The products of these reactions were intact messenger or genome-sized RNA species. A preparation from HeLa cells containing tubulin and microtubule-associated proteins also stimulated vesicular stomatitis virus transcription in vitro. This observation is in accord with previous studies, which suggested that a host cell factor was involved with the function of the vesicular stomatitis virus RNA polymerase, and others that indicated that several animal viruses displayed an association with host cell cytoskeletal elements during their replication cycles. We show evidence in this report of a host cell protein that seems to have a functional role in interacting with the virion polymerase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Luftig R. B., Weatherbee J. A., Weihing R. R., Ray U. R., Fields B. N. Reovirus serotypes 1 and 3 differ in their in vitro association with microtubules. J Virol. 1979 Jun;30(3):863–874. doi: 10.1128/jvi.30.3.863-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Self-assembly of microtubules in extracts of cultured HeLa cells and the identification of HeLa microtubule-associated proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):293–297. doi: 10.1073/pnas.76.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Cervera M. M., Penman S. Formation of vesicular stomatitis virus nucleocapsid from cytoskeletal framework-bound N protein: possible model for structure assembly. Mol Cell Biol. 1984 Oct;4(10):2231–2234. doi: 10.1128/mcb.4.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Egly J. M., Miyamoto N. G., Moncollin V., Chambon P. Is actin a transcription initiation factor for RNA polymerase B? EMBO J. 1984 Oct;3(10):2363–2371. doi: 10.1002/j.1460-2075.1984.tb02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Nishikawa K., Toyoda T., Yoshida T., Hanaichi T., Nagai Y. Transcriptive complex of Newcastle disease virus. II. Structural and functional assembly associated with the cytoskeletal framework. Virology. 1985 Dec;147(2):295–308. doi: 10.1016/0042-6822(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Harmon S. A., Summers D. F. Characterization of monospecific antisera against all five vesicular stomatitis virus-specific proteins: anti-L and anti-NS inhibit transcription in vitro. Virology. 1982 Jul 15;120(1):194–204. doi: 10.1016/0042-6822(82)90017-4. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Marnell L., Summers D. F. In vitro replication and assembly of vesicular stomatitis virus nucleocapsids. Virology. 1981 Aug;113(1):109–118. doi: 10.1016/0042-6822(81)90140-9. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Summers D. F. Synthesis of VSV RNPs in vitro by cellular VSV RNPs added to uninfected HeLa cell extracts: VSV protein requirements for replication in vitro. Virology. 1982 Dec;123(2):407–419. doi: 10.1016/0042-6822(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Inouye H., Pollack Y., Petre J. Physical and functional homology between ribosomal protein S1 and interference factor i. Eur J Biochem. 1974 Jun 1;45(1):109–117. doi: 10.1111/j.1432-1033.1974.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Luftig R. B. Does the cytoskeleton play a significant role in animal virus replication? J Theor Biol. 1982 Nov 7;99(1):173–191. doi: 10.1016/0022-5193(82)90397-6. [DOI] [PubMed] [Google Scholar]
- Nowakowski M., Bloom B. R., Ehrenfeld E., Summers D. F. Restricted replication of vesicular stomatitis virus in human lymphoblastoid cells. J Virol. 1973 Dec;12(6):1272–1278. doi: 10.1128/jvi.12.6.1272-1278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Keene J. D. Replication of the vesicular stomatitis virus genome in permissive and nonpermissive host cells. J Biol Chem. 1985 Sep 5;260(19):10503–10511. [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. The tdCE and hrCE phenotypes: host range mutants of vesicular stomatitis virus in which polymerase function is affected. Cell. 1978 Oct;15(2):597–606. doi: 10.1016/0092-8674(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Obijeski J. F. Conditional lethal mutants of vesicular stomatitis virus. I. Phenotypic characterization of single and double mutants exhibiting host restriction and temperature sensitivity. Virology. 1974 Feb;57(2):357–368. doi: 10.1016/0042-6822(74)90175-5. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Obijeski J. F., Morrongiello M. P. Conditional lethal mutants of vesicular stomatitis virus. III. Host range properties, interfering capacity, and complementation patterns of specific hr mutants. Virology. 1979 Mar;93(2):493–505. doi: 10.1016/0042-6822(79)90252-6. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Abortive infection of a rabbit cornea cell line by vesicular stomatitis virus: conversion to productive infection by superinfection with vaccinia virus. J Virol. 1975 Aug;16(2):322–329. doi: 10.1128/jvi.16.2.322-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff S. N., Grisham L. M. In vitro assembly of cytoplasmic microtubules. Annu Rev Biochem. 1980;49:565–591. doi: 10.1146/annurev.bi.49.070180.003025. [DOI] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- Weatherbee J. A., Luftig R. B., Weihing R. R. Binding of adenovirus to microtubules. II. Depletion of high-molecular-weight microtubule-associated protein content reduces specificity of in vitro binding. J Virol. 1977 Feb;21(2):732–742. doi: 10.1128/jvi.21.2.732-742.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed H. G., Krochmalnic G., Penman S. Poliovirus metabolism and the cytoskeletal framework: detergent extraction and resinless section electron microscopy. J Virol. 1985 Nov;56(2):549–557. doi: 10.1128/jvi.56.2.549-557.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]