Abstract
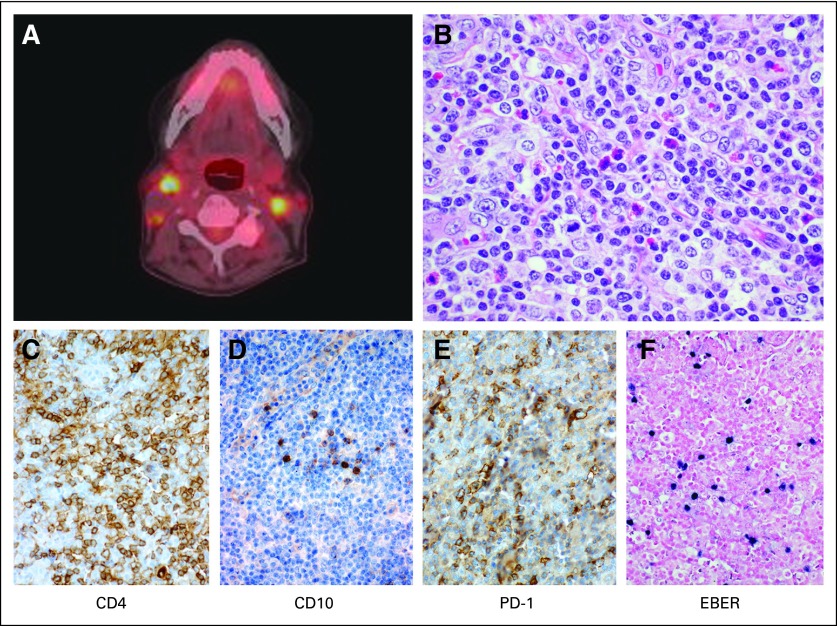
A 69-year-old woman was referred for further evaluation and management of relapsed angioimmunoblastic T-cell lymphoma. At diagnosis, she received six cycles of dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) and achieved a complete response (CR). Her first surveillance computed tomography scan 3 months later demonstrated enlarging cervical lymphadenopathy. A lymph node excision confirmed relapsed angioimmunoblastic T-cell lymphoma with atypical lymphocytes expressing CD3, CD4, CD10, PD-1, and EBER, with loss of CD5 (Fig 1). A clonal T-cell receptor beta and gamma rearrangement by polymerase chain reaction was identical to that in her initial diagnostic biopsy. At our initial consultation, options for standard as well as investigational therapies were discussed, and HLA typing was initiated. The patient was enrolled onto an investigational phase II study; however, she developed progressive disease after two cycles. She was then treated with romidepsin 14 mg/m2 administered intravenously for 3 consecutive weeks with 1 week off. After two cycles, she achieved a partial response, and after four additional cycles, she maintained her response without further improvement. We discussed additional treatment options.
The Oncology Grand Rounds series is designed to place original reports published in the Journal into clinical context. A case presentation will be followed by a description of diagnostic and management challenges, a review of the relevant literature, and a summary of the authors' suggested management approaches. The goal of this series is to help readers better understand how to apply the results of key studies, including those published in Journal of Clinical Oncology, to patients seen in their own clinical practice.
CHALLENGES IN DIAGNOSIS AND MANAGEMENT
Nearly two decades ago, the Revised European-American Lymphoma classification formally differentiated B- and T-cell lymphomas.1 Peripheral T-cell lymphomas (PTCLs) are malignancies arising from mature or post-thymic T lymphocytes. PTCL represents approximately 10% of all new diagnoses of non-Hodgkin lymphoma.2 Despite the infrequency, PTCLs are heterogeneous malignancies with 22 described clinicopathologic subtypes.3 The subtypes PTCL–not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large-cell lymphoma (ALCL) represent the three most common entities, accounting for almost 75% of patient cases in North America and Europe.4 According to the International Peripheral T-Cell Lymphoma Project (the largest retrospective series), 5-year overall survival (OS) for PTCL-NOS, AITL, ALK-negative ALCL, and ALK-positive ALCL are 32%, 32%, 49%, and 70%, respectively.
There is no universally agreed-on standard first-line regimen in PTCL; however, for the most common subtypes, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) is frequently used. The overall response rate (ORR) to CHOP may be as high as 79%, with 39% CRs; however, durable remissions after CHOP alone are uncommon, with < 30% of patients progression free at 5 years.5–7 The addition of etoposide to CHOP (CHOEP) has been studied by the German High-Grade Non-Hodgkin Lymphoma Study Group and most recently by the Nordic Lymphoma Group as part of a first-line autologous strategy.8,9 In the Nordic study, CHOEP had an ORR of 82%, with 51% attaining a CR and 70% responding adequately enough to move forward to consolidative stem-cell transplantation. Multiple alternative regimens to CHOP have been studied, but none are clearly superior.7,10–13 Consolidative transplantation strategies remain an appealing option in first remission.5,9,14–16
For those with primary refractory or relapsed PTCL, the optimal approach to management is unclear, and data regarding the outcome for these patients is limited. A common paradigm is to treat with second-line combination regimens similar to those studied in relapsed aggressive B-cell lymphomas. Although earlier studies of these regimens, such as ICE (ifosphamide, carboplatin, and etoposide), DHAP (dexamethasone, cytarabine, and cisplatin), and ESHAP (etoposide, methylprednisolone, cisplatin, and cytarabine), included patients with T-cell lymphoma, the T-cell lymphoma subsets have never been identified or retrospectively analyzed.17–20
SUMMARY OF THE RELEVANT LITERATURE
In the report accompanying this article, Mak et al21 present the outcomes for patients with relapsed and refractory PTCL-NOS, AITL, and ALCL treated at the British Columbia Cancer Agency (BCCA) from 1976 to 2010. This represents the largest reported series of relapsed and refractory disease for the most common subtypes of PTCL. This study excluded those who proceeded to hematopoietic stem-cell transplantation, and the study found few long-term survivors. Of the 153 patients in the series, the median OS was 5.5 months. For the subset of patients in this series who received treatment, the median OS was only marginally longer at 6.5 months. The treatment strategies reported are typical of those used for relapsed lymphoma, with 91 patients (58%) receiving chemotherapy, including 46% as part of a multidrug regimen.
Until recently, our understanding of the prognosis for patients was gleaned from small phase II clinical trials where the reports are focused on response rates with little information on OS (Table 1).22–26a Large phase II studies have now been completed, providing valuable information regarding the prognosis for this patient population. The phase II studies for romidepsin and pralatrexate enrolled 130 and 111 patients, respectively, and led to the approval of these drugs in relapsed and refractory PTCLs.27–28a Interestingly, we see apparent differences in outcomes in these large phase II studies compared with the BCCA series. In the two studies, the ORR was 29% for pralatrexate and 25% for romidepsin, with median OS of 14.5 and 11.3 months, respectively. These survival figures are double that seen in the BCCA series, and it seems that the tails of those curves show more patients alive beyond 2 and 3 years. It can be perilous to draw conclusions by comparing phase II clinical trial results with population-based registry outcomes. However, in a disease where we lack randomized studies, such are the data we have to help guide decisions. What could account for the different outcomes? Patient selection is one likely contribution. Patients in trials tend to be in better shape. Most had Eastern Cooperative Oncology Group performance status (PS) of 0 to 1, whereas PS was ≥ 2 in 50% of the historical controls. In addition to PS, the populations differed by prior therapy. The BCCA patients were described from first relapse, whereas those in the prospective studies were enrolled after a median of 2 to 3 prior therapies. The patients in the clinical trials were further along in their disease courses (> 15 months from diagnosis in both pralatrexate and romidepsin studies v 6.6 months from diagnosis in the BCCA series) but still showed longer survival. Another possibility is that the new drugs are actually more effective. They are certainly better studied, but a conclusion that they are more active is hard to support when their ORRs were approximately 25% to 30%, and the ORR for all therapies reported by Mak et al21 was 55%.
Table 1.
Studies Exclusively in Relapsed PTCL
| Study | No. of Patients | ORR (%) | CR (%) | PFS (months) | DOR (months) | OS (months) |
|---|---|---|---|---|---|---|
| BCCA series | 153 | 55 | 26 | 3.1 | NR | 6.5 |
| Romidepsin† | 130 | 25 | 15 | 4 | 28 | 11.3 |
| Pralatrexate | 111 | 29 | 11 | 3.5 | 10.1 | 14.5 |
| Bendamustine | 60 | 50 | 28 | 3.6 | 3.5 | 6.2 |
| Denileukin diftitox* | 27 | 48 | 22 | 6 | NR | NR |
| Lenalidomide | 23 | 30 | 0 | 3 | NR | 8 |
| Alemtuzumab | 14 | 36 | 14 | NR | NR | NR |
Abbreviations: BCCA, British Columbia Cancer Agency; CR, complete response; DOR, duration of response; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PTCL, peripheral T-cell lymphoma.
No longer available.
DOR, PFS, and OS are from updated data.
A third distinction might be the difference between short-course combination versus continuous therapy. We know that remissions while not receiving therapy are often short in PTCLs, even in the first-line setting. In the studies of the new agents, because of study design and lack of cumulative toxicity, patients were able to be treated until progression or intolerance so that responding patients maintained their remissions. We see the potential benefits of this approach in the median durations of response: pralatrexate, 10.1 months; romidepsin, 28 months; and brentuximab vedotin, 13 months (ALCL only).29 In these trials, excluding that involving brentuximab vedotin, where therapy was capped at 1 year, patients who did not experience progression could continue therapy, and they may have had their disease control extended by this approach. Combination chemotherapy with non–cross-reactive regimens DHAP, ICE, ESHAP, Gem-P (gemcitabine, cisplatin, and methylprednisolone), and GCD (gemcitabine, cisplatin, and dexamethasone) has traditionally been used.18–20,30,31 However, there are few published data for these regimens in PTCL. Combination chemotherapy regimens may result in higher response rates, but because of cumulative toxicity, they are usually only administered for three to four cycles. This may work well as a bridge to stem-cell transplantation, but it lacks durability as a standalone option. For example, in our experience with ICE as second-line therapy, we found an ORR of 70% among the 40 patients we treated; however, despite two thirds of these patients preceding to autologous stem-cell transplantation (ASCT), our median progression-free survival was < 6 months.32 In a study of Gem-P for relapsed PTCL, an ORR of 69% was seen in 16 patients; however, the time to progression was only 4 months.30 A recent example of the potential benefits of continuous versus interrupted therapy for relapsed PTCL comes from a trial of bendamustine.33 In that study, 60 patients with relapsed PTCL were treated with bendamustine, with an ORR of 50%. Despite the higher response rate as compared with pralatrexate and romidepsin, the median duration of response was only 3.5 months, and the median OS was 6.2 months. Most patients received < four cycles of therapy.
It is important to note that the use of transplantation in our more-current treatment paradigms may be holding up the tails of the curves. Our institutional data and others have shown that the use of ASCT for relapsed PTCL, with a possible exception of ALCL, has rarely resulted in long-term disease control.32,34 This is somewhat controversial, and some registry data point to better results with ASCT at relapse, although these series are overrepresented by ALCL.35 Meanwhile, the emerging experience with allogeneic transplantation looks promising. Both myeloablative and reduced-intensity allogeneic stem-cell transplantation have demonstrated up to 60% 3-year progression-free survival.36–38 In the BCCA series, only 29% of patients at relapse were felt to be transplantation eligible. However, this series spans more than three decades, and in the current era of reduced-intensity transplantation, the definition of transplantation eligible is surely much broader. As more patients who respond to therapy at relapse are cured with allogeneic stem-cell transplantation, the tails of the curves are sure to be extended.
Clinical trials remain an integral part of the care of patients with relapsed PTCL. Agents in development are initially studied in the relapse setting and most often follow the paradigm set forth by pralatrexate and romidepsin of disease control and maintenance of a response. Currently, there are several single agents in development for relapsed PTCL, and until highly effective therapies are developed, participation in a clinical trial should be strongly considered whenever a new line of therapy is needed (Table 2).
Table 2.
Pipeline Single Agents in Relapsed PTCL
| Agent | NCT No. | Study | Mechanism of Action |
|---|---|---|---|
| Alisertib (MLN-8237) | 01466881 | Alisertib in treating patients with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma | Aurora kinase A inhibitor |
| Mogamulizumab (KW-0761) | 00888927 | Safety study to evaluate monoclonal antibody KW-0761 in patients with PTCL | Dufucosylated anti-CCR4 monoclonal antibody |
| Brentuximab vedotin (SGN-35) | 01421667 | Study of brentuximab vedotin in relapsed/refractory CD30+ non-Hodgkin lymphoma | CD30 antibody drug conjugate to monomethyl auristatin E |
| Belinostat (PXD 101) | 00865969 | Belinostat in relapsed/ refractory PTCL | Histone deacetylase inhibitor |
| Carfilzomib | 01336920 | Carfilzomib in treating patients with relapsed or refractory T-cell lymphoma | Proteasome inhibitor |
Abbreviations: NCT, national clinical trial; PTCL, peripheral T-cell lymphoma.
SUGGESTED APPROACHES TO MANAGEMENT
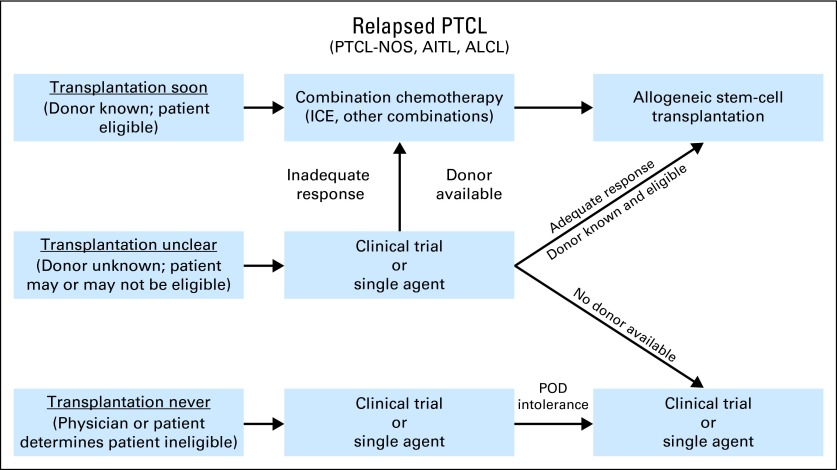
Without comparative data, our practice patterns are informed by the available literature and our personal experience. For the purposes of creating an algorithmic approach, our general assumptions are that in the relapsed setting, allogeneic transplantation is the only reliably curative approach, and outside of a curative approach, the best chance at achieving a durable remission is through a continuous treatment approach. On the basis of these assumptions, patients with relapsed disease can be subdivided into three basic groups with regard to their potential for curative therapy: transplantation soon, transplantation never, or transplantation unclear, with the majority falling into this last category (Fig 2).
Fig 2.
Recommended approach to patients with relapsed peripheral T-cell lymphomas (PTCLs) regarding additional therapies and goals of care. AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ICE, ifosphamide, carboplatin, and etoposide; NOS, not otherwise specified; POD, progression of disease.
Fig 1.
(A) Transverse section imaging by positron emission tomography/computer tomography demonstrating avid bilateral cervical lymph nodes. (B) Subsequent lymph node excision biopsy with corresponding hematoxylin and eosin stain as well as immunophenotyping ([C] CD4; [D] CD10; [E] PD-1; [F] EBER) confirmed the diagnosis of angioimmunoblastic T-cell lymphoma.
Transplantation Soon
Candidates for early transplantation include those without significant comorbidities and with a known donor identified and available. The treatment goal is to achieve a quick remission and then consolidation with allogeneic stem-cell transplantation. The situations where autologous transplantation may be considered curative, such as relapsed ALK-positive ALCL, could be included here. We believe combination chemotherapy with common second-line regimens such as ICE (our preferred choice if relapse is after CHOP), ESHAP, or DHAP or others offers the highest chance of inducing both prompt and often complete remission. This allows the patient to proceed to transplantation after two to three cycles of second-line therapy. Because patients with PTCL have a propensity to relapse quickly when not receiving therapy, we try to avoid delays between second-line therapy and the conditioning regimen and consequently reserve this initial approach for those who already have an identified donor. Even in these cases, organizing the transplantation plan must be expedited. If, for example, three cycles of ICE are administered every 17 to 21 days, this means that a patient should be ready to be admitted for transplantation < 10 weeks from day 1 of his or her first ICE treatment.
Transplantation Never
We categorize here patients whose comorbidities or personal choices eliminate curative therapy as an option. Historically, age (with definitions changing over time) and lack of an HLA-matched donor could also be reasons to include someone in this category. However, the increasing use of reduced-intensity transplantation and alternate stem-cell sources make this group more challenging to define. We frequently consult with our transplantation service before assigning individuals to this group. Without transplantation, the therapeutic goal is to maintain remission. We treat with single agents and well-tolerated combinations, with the goal of achieving disease control and maintaining as good a quality of life as possible for as long as possible while administering therapy. Currently, outside of brentuximab vedotin for relapsed ALCL, the data for the available single agents are insufficient to endorse one over another as first choice in this setting. Rather, schedule and administration, potential adverse effects, previous therapy, and physician comfort in addition to patient preferences often guide the choice, because all these agents have response rates < 50%. Choice of therapy at relapse becomes less about picking the best agent to use and more about organizing potential treatments in order of which to try first, second, third, and so on. By using this sequential approach and capitalizing on our increasing number of active therapies for PTCL, a significant subset of patients can have their disease controlled to surpass the median survival times described in the series by the BCCA. This is also an opportune place to incorporate clinical trials, because there are a number of novel drugs in development, including oral agents and antibodies, that fit this paradigm.
Transplantation Unclear
In the transplantation-unclear group, which in our experience is the largest subset, comprising approximately two thirds of our relapsed PTCL population, we use a hybrid of the two approaches described. At time of relapse for a patient who is a potential transplantation candidate, we initiate HLA typing and a transplantation consultation concurrently with planning therapy. In these cases, we generally start therapy with one of the single agents or mild combinations therapies that can be continued. We have a strong bias toward investigational therapies in this setting. If a response is achieved, and a transplantation plan is made, patients can transition directly to transplantation, as we have seen in the phase II studies of pralatrexate, romidepsin, and brentuximab vedotin. If a response is achieved, and a transplantation option does not materialize, the patient needs time to consider his or her preferences, or, as is often the case with matched unrelated donors, it takes some time to organize transplantation, the patient can continue to receive therapy until things are in place. This approach avoids the quickly ticking clock associated with the more-aggressive second-line regimens that carry a higher risk of cumulative toxicity after several cycles. If a response to the investigational agent or single agent is not seen, and a transplantation plan is set, the patient can then be transitioned to one of the combination regimens to try to induce a prompt remission and move to transplantation. If a response is not seen, and no transplantation plan is in place, we generally offer an alternate single agent or alternate investigational agent.
Mak et al21 provide valuable information regarding the prognosis for patients with relapsed PTCL. With newer agents now available, such as romidepsin, pralatrexate, and brentuximab vedotin, and others in development, a greater proportion of relapsed patients will have longer disease control, raising and extending the tails of these survival curves. Ultimately, more-effective first-line regimens will make discussions about the tails of the curves unnecessary. However, until that time, strategies that integrate clinical trials, sequential treatment with less toxic, better-tolerated agents, and selective use of allogeneic stem-cell transplantation seem to be the best ways we have of extending survival.
After much discussion, our patient elected to proceed to reduced-intensity matched unrelated donor stem-cell transplantation. She obtained a complete remission at her first post-transplantation evaluation. She is currently 2 years post-transplantation without evidence of disease, with grade 2 chronic graft-versus-host disease of the skin.
Footnotes
See accompanying article on page 1970
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Steven Horwitz, Celgene (C), Allos Therapeutics (C), Seattle Genetics (C), Bristol-Myers Squibb (C), Genzyme (C), Kyowa Hakko Kirin Pharma (C), Janssen (C), Millennium Pharmaceuticals (C), Hospira (C) Stock Ownership: None Honoraria: None Research Funding: Steven Horwitz, Celgene, Allos Therapeutics, Seattle Genetics, Infinity Pharmaceuticals, Kyowa Hakko Kirin Pharma, Millennium Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Harris N, Jaffe E, Stein H. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1631–1692. [PubMed] [Google Scholar]
- 2.Savage KJ. Peripheral T-cell lymphomas. Blood Rev. 2007;21:201–216. doi: 10.1016/j.blre.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, et al., editors. Lyon, France: IARC Press; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 4.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 5.Reimer P, Rüdiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: Results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 6.Savage KJ, Chhanabhai M, Gascoyne RD, et al. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- 7.Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma: Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151:159–166. doi: 10.1111/j.1365-2141.2010.08329.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 9.d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 10.Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, et al. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol. 2011;22:1595–1600. doi: 10.1093/annonc/mdq635. [DOI] [PubMed] [Google Scholar]
- 11.Mahadevan D, Unger JM, Spier CM, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2013;119:371–379. doi: 10.1002/cncr.27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallamini A, Zaja F, Patti C, et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–2323. doi: 10.1182/blood-2007-02-074641. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Yoon DH, Kang HJ, et al. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: A multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48:3223–3231. doi: 10.1016/j.ejca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez J, Conde E, Gutiérrez A, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: A prospective study from the Gel-Tamo Study Group. Eur J Haematol. 2007;79:32–38. doi: 10.1111/j.1600-0609.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 15.Corradini P, Tarella C, Zallio F, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–1538. doi: 10.1038/sj.leu.2404306. [DOI] [PubMed] [Google Scholar]
- 16.Mercadal S, Briones J, Xicoy B, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19:958–963. doi: 10.1093/annonc/mdn022. [DOI] [PubMed] [Google Scholar]
- 17.Jerkeman M, Leppä S, Kvaløy S, et al. ICE (ifosfamide, carboplatin, etoposide) as second-line chemotherapy in relapsed or primary progressive aggressive lymphoma: The Nordic Lymphoma Group experience. Eur J Haematol. 2004;73:179–182. doi: 10.1111/j.1600-0609.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- 18.Philip T, Chauvin F, Armitage J, et al. Parma international protocol: Pilot study of DHAP followed by involved-field radiotherapy and BEAC with autologous bone marrow transplantation. Blood. 1991;77:1587–1592. [PubMed] [Google Scholar]
- 19.Zelenetz AD, Hamlin P, Kewalramani T, et al. Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin's lymphoma. Ann Oncol. 2003;14(suppl 1):i5–i10. doi: 10.1093/annonc/mdg702. [DOI] [PubMed] [Google Scholar]
- 20.Velasquez WS, McLaughlin P, Tucker S, et al. ESHAP: An effective chemotherapy regimen in refractory and relapsing lymphoma—A 4-year follow-up study. J Clin Oncol. 1994;12:1169–1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 21.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: Spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 22.Zinzani PL, Venturini F, Stefoni V, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: Evaluation of the long-term outcome. Ann Oncol. 2010;21:860–863. doi: 10.1093/annonc/mdp508. [DOI] [PubMed] [Google Scholar]
- 23.Czuczman MS, Porcu P, Johnson J, et al. Results of a phase II study of 506U78 in cutaneous T-cell lymphoma and peripheral T-cell lymphoma: CALGB 59901. Leuk Lymphoma. 2007;48:97–103. doi: 10.1080/10428190600961058. [DOI] [PubMed] [Google Scholar]
- 24.Dearden C, Matutes E, Catovsky D. Deoxycoformycin in the treatment of mature T-cell leukaemias. Br J Cancer. 1991;64:903–906. doi: 10.1038/bjc.1991.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dueck G, Chua N, Prasad A, et al. Interim report of a phase 2 clinical trial of lenalidomide for T-cell non-Hodgkin lymphoma. Cancer. 2010;116:4541–4548. doi: 10.1002/cncr.25377. [DOI] [PubMed] [Google Scholar]
- 26.Dang NH, Pro B, Hagemeister FB, et al. Phase II trial of denileukin diftitox for relapsed/refractory T-cell non-Hodgkin lymphoma. Br J Haematol. 2007;136:439–447. doi: 10.1111/j.1365-2141.2006.06457.x. [DOI] [PubMed] [Google Scholar]
- 26a.Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103:2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 27.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Coiffier B, Pro B, Prince M, et al. Romidepsin induces durable responses in patients with peripheral T-cell lymphoma: GPI-06-0002 study update. 54th Annual Meeting of the American Society of Hematology; December 8-11, 2012; Atlanta, GA. abstr 3641. [Google Scholar]
- 29.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: Results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 30.Arkenau HT, Chong G, Cunningham D, et al. Gemcitabine, cisplatin and methylprednisolone for the treatment of patients with peripheral T-cell lymphoma: The Royal Marsden Hospital experience. Haematologica. 2007;92:271–272. doi: 10.3324/haematol.10737. [DOI] [PubMed] [Google Scholar]
- 31.Crump M, Shepherd L, Lin B. A randomized phase III study of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin as salvage chemotherapy followed by posttransplantation rituximab maintenance therapy versus observation for treatment of aggressive B-cell and T-cell non-Hodgkin's lymphoma. Clin Lymphoma. 2005;6:56–60. doi: 10.3816/clm.2005.n.030. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz SMC, Kewalramani T, et al. Second-line therapy with ICE followed by high dose therapy and autologous stem cell transplantation for relapsed/refractory peripheral T-cell lymphomas: Minimal benefit when analyzed by intent to treat. Blood. 2005:106. abstr 2679. [Google Scholar]
- 33.Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: The BENTLY trial. J Clin Oncol. 2013;31:104–110. doi: 10.1200/JCO.2012.43.7285. [DOI] [PubMed] [Google Scholar]
- 34.Smith SD, Bolwell BJ, Rybicki LA, et al. Autologous hematopoietic stem cell transplantation in peripheral T-cell lymphoma using a uniform high-dose regimen. Bone Marrow Transplant. 2007;40:239–243. doi: 10.1038/sj.bmt.1705712. [DOI] [PubMed] [Google Scholar]
- 35.Smith SBL, van Biesen K, LeRademacher J, et al. Autologous (auto) versus allogeneic (allo) hematopoietic cell transplantation (HCT) for T-NHL: A CIBMTR analysis. Blood. 2010:116. abstr 689. [Google Scholar]
- 36.Le Gouill S, Milpied N, Buzyn A, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: A study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen ED, Kim HT, Ho VT, et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011;22:1608–1613. doi: 10.1093/annonc/mdq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg JD, Chou JF, Horwitz S, et al. Long-term survival in patients with peripheral T-cell non-Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2012;53:1124–1129. doi: 10.3109/10428194.2011.645818. [DOI] [PMC free article] [PubMed] [Google Scholar]