Abstract
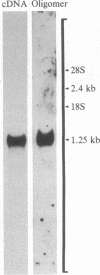
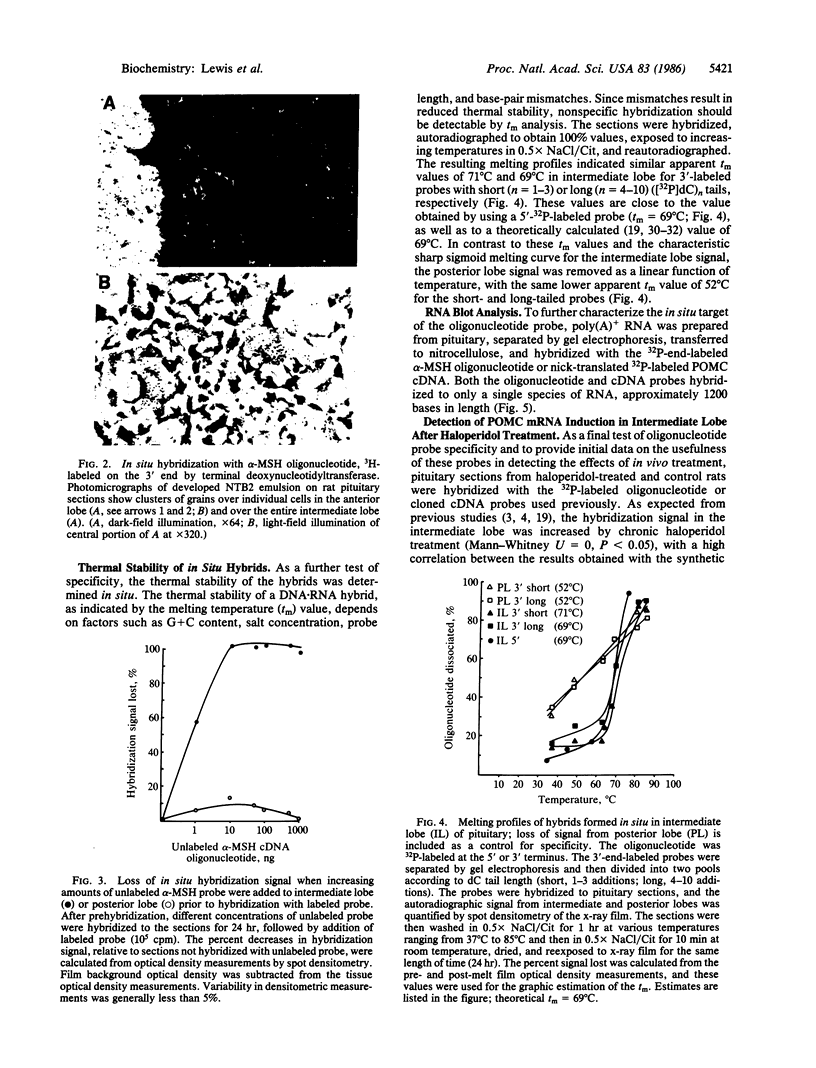
Synthetic oligonucleotide probes can be easily obtained and used, in contrast to cDNA cloning to develop probes, and thus the present study was carried out to determine whether such probes could also be useful for in situ hybridization. A 24-base synthetic oligonucleotide complementary to part of the alpha-melanocyte-stimulating hormone (alpha-MSH) coding region of proopiomelanocortin (POMC) mRNA was 5'-end-labeled by using [gamma-32P]ATP with T4 polynucleotide kinase or was 3' tailed by using [alpha-32P]dATP or [3H]dCTP with terminal deoxynucleotidyltransferase. Blot analysis of pituitary poly(A)+ RNA showed that the oligonucleotide hybridized to a single species with a molecular size of approximately 1200 nucleotides, consistent with that determined previously for POMC mRNA. The oligonucleotide, regardless of labeling method, hybridized to cells in the pituitary intermediate lobe, but not in the posterior lobe. Only the 3H-labeled probe gave resolution of individual pituitary anterior lobe cells. The specificity of the hybridization was determined by showing that the intermediate lobe signal was blocked by prehybridization of the tissue with unlabeled alpha-MSH oligonucleotide probe. Furthermore, the hybridized probe exhibited a sharp sigmoid curve when melted off. Finally, the oligonucleotide probe detected, in situ, the haloperidol-induced elevation of intermediate lobe POMC mRNA. Thus, the oligonucleotide probe exhibited hybridization in an anatomically and biochemically specific manner, and it detected a tissue-specific change in mRNA levels in situ.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentzen R., Baldino F., Jr, Davis L. G., Higgins G. A., Lin Y., Manning R. W., Wolfson B. In situ hybridization of putative somatostatin mRNA within hypothalamus of the rat using synthetic oligonucleotide probes. J Cell Biochem. 1985;27(4):415–422. doi: 10.1002/jcb.240270410. [DOI] [PubMed] [Google Scholar]
- Birnberg N. C., Lissitzky J. C., Hinman M., Herbert E. Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6982–6986. doi: 10.1073/pnas.80.22.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch B., Milner R. J., Baird A., Gubler U., Reymond C., Bohlen P., le Guellec D., Bloom F. E. Detection of the messenger RNA coding for preproenkephalin A in bovine adrenal by in situ hybridization. Regul Pept. 1984 Jul;8(4):345–354. doi: 10.1016/0167-0115(84)90045-4. [DOI] [PubMed] [Google Scholar]
- Bloom F., Battenberg E., Rossier J., Ling N., Leppaluoto J., Vargo T. M., Guillemin R. Endorphins are located in the intermediate and anterior lobes of the pituitary gland, not in the neurohypophysis. Life Sci. 1977 Jan 1;20(1):43–47. doi: 10.1016/0024-3205(77)90126-6. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Dionne F. T., Roberts J. L. Regulation of the pro-opiomelanocortin mRNA levels in rat pituitary by dopaminergic compounds. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2211–2215. doi: 10.1073/pnas.80.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Civelli O., Birnberg N., Comb M., Douglass J., Lissitzky J. C., Uhler M., Herbert E. Regulation of opioid gene expression. Peptides. 1983 Sep-Oct;4(5):651–656. doi: 10.1016/0196-9781(83)90013-x. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Dauth G. W., Frey K. A., Gilman S. A densitometer for quantitative autoradiography. J Neurosci Methods. 1983 Nov;9(3):243–251. doi: 10.1016/0165-0270(83)90087-0. [DOI] [PubMed] [Google Scholar]
- Davis L. G., Arentzen R., Reid J. M., Manning R. W., Wolfson B., Lawrence K. L., Baldino F., Jr Glucocorticoid sensitivity of vasopressin mRNA levels in the paraventricular nucleus of the rat. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1145–1149. doi: 10.1073/pnas.83.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H. J., Belagaje R., Brown E. L., Fritz R. H., Jones R. A., Lees R. G., Khorana H. G. High-pressure liquid chromatography in polynucleotide synthesis. Biochemistry. 1978 Apr 4;17(7):1257–1267. doi: 10.1021/bi00600a020. [DOI] [PubMed] [Google Scholar]
- Fuller P. J., Clements J. A., Funder J. W. Localization of arginine vasopressin-neurophysin II messenger ribonucleic acid in the hypothalamus of control and Brattleboro rats by hybridization histochemistry with a synthetic pentadecamer oligonucleotide probe. Endocrinology. 1985 Jun;116(6):2366–2368. doi: 10.1210/endo-116-6-2366. [DOI] [PubMed] [Google Scholar]
- Gee C. E., Chen C. L., Roberts J. L., Thompson R., Watson S. J. Identification of proopiomelanocortin neurones in rat hypothalamus by in situ cDNA-mRNA hybridization. Nature. 1983 Nov 24;306(5941):374–376. doi: 10.1038/306374a0. [DOI] [PubMed] [Google Scholar]
- Gee C. E., Roberts J. L. In situ hybridization histochemistry: a technique for the study of gene expression in single cells. DNA. 1983;2(2):157–163. doi: 10.1089/dna.1983.2.157. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Griffin W. S., Alejos M., Nilaver G., Morrison M. R. Brain protein and messenger RNA identification in the same cell. Brain Res Bull. 1983 May;10(5):597–601. doi: 10.1016/0361-9230(83)90027-8. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Walker D., Stowring L., Ventura P., Geballe A., Blum H., Brahic M., Goldberg R., O'Brien K. Detection of two viral genomes in single cells by double-label hybridization in situ and color microradioautography. Science. 1985 Jan 11;227(4683):189–192. doi: 10.1126/science.2981430. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Conkie D., Paul J., Jones K. Localisation of cellular globin messenger RNA by in situ hybridisation to complementary DNA. FEBS Lett. 1973 May 15;32(1):109–112. doi: 10.1016/0014-5793(73)80749-5. [DOI] [PubMed] [Google Scholar]
- Hudson P., Penschow J., Shine J., Ryan G., Niall H., Coghlan J. Hybridization histochemistry: use of recombinant DNA as a "homing probe" for tissue localization of specific mRNA populations. Endocrinology. 1981 Jan;108(1):353–356. doi: 10.1210/endo-108-1-353. [DOI] [PubMed] [Google Scholar]
- Höllt V., Haarmann I., Seizinger B. R., Herz A. Chronic haloperidol treatment increases the level of in vitro translatable messenger ribonucleic acid coding for the beta-endorphin/adrenocorticotropin precursor proopiomelanocortin in the pars intermedia of the rat pituitary. Endocrinology. 1982 Jun;110(6):1885–1891. doi: 10.1210/endo-110-6-1885. [DOI] [PubMed] [Google Scholar]
- Kelsey J. E., Watson S. J., Burke S., Akil H., Roberts J. L. Characterization of proopiomelanocortin mRNA detected by in situ hybridization. J Neurosci. 1986 Jan;6(1):38–42. doi: 10.1523/JNEUROSCI.06-01-00038.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. E., Sherman T. G., Watson S. J. In situ hybridization histochemistry with synthetic oligonucleotides: strategies and methods. Peptides. 1985;6 (Suppl 2):75–87. doi: 10.1016/0196-9781(85)90138-x. [DOI] [PubMed] [Google Scholar]
- Majzoub J. A., Rich A., van Boom J., Habener J. F. Vasopressin and oxytocin mRNA regulation in the rat assessed by hybridization with synthetic oligonucleotides. J Biol Chem. 1983 Dec 10;258(23):14061–14064. [PubMed] [Google Scholar]
- McWilliams D., Boime I. Cytological localization of placental lactogen messenger ribonucleic acid in syncytiotrophoblast layers of human placenta. Endocrinology. 1980 Sep;107(3):761–765. doi: 10.1210/endo-107-3-761. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nojiri H., Sato M., Urano A. In situ hybridization of the vasopressin mRNA in the rat hypothalamus by use of a synthetic oligonucleotide probe. Neurosci Lett. 1985 Jul 4;58(1):101–105. doi: 10.1016/0304-3940(85)90336-2. [DOI] [PubMed] [Google Scholar]
- Pelletier G., Leclerc R., Labrie F., Cote J., Chretien M., Lis M. Immunohistochemical localization of beta-lipotropic hormone in the pituitary gland. Endocrinology. 1977 Mar;100(3):770–776. doi: 10.1210/endo-100-3-770. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Pochet R., Brocas H., Vassart G., Toubeau G., Seo H., Refetoff S., Dumont J. E., Pasteels J. L. Radioautographic localization of prolactin messenger RNA on histological sections by in situ hybridization. Brain Res. 1981 May 4;211(2):433–438. doi: 10.1016/0006-8993(81)90969-0. [DOI] [PubMed] [Google Scholar]
- Schachter B. S., Johnson L. K., Baxter J. D., Roberts J. L. Differential regulation by glucocorticoids of proopiomelanocortin mRNA levels in the anterior and intermediate lobes of the rat pituitary. Endocrinology. 1982 Apr;110(4):1442–1444. doi: 10.1210/endo-110-4-1442. [DOI] [PubMed] [Google Scholar]
- Sherman T. G., McKelvy J. F., Watson S. J. Vasopressin mRNA regulation in individual hypothalamic nuclei: a northern and in situ hybridization analysis. J Neurosci. 1986 Jun;6(6):1685–1694. doi: 10.1523/JNEUROSCI.06-06-01685.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Ward D. C. Actin gene expression visualized in chicken muscle tissue culture by using in situ hybridization with a biotinated nucleotide analog. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7331–7335. doi: 10.1073/pnas.79.23.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G. R., Zingg H. H., Habener J. F. Vasopressin mRNA in situ hybridization: localization and regulation studied with oligonucleotide cDNA probes in normal and Brattleboro rat hypothalamus. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5555–5559. doi: 10.1073/pnas.82.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson B., Manning R. W., Davis L. G., Arentzen R., Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985 May 2;315(6014):59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]