Abstract
Objectives
The San Francisco Department of Public Health (SFDPH) has the goal of offering HIV partner services (PS) to all individuals newly diagnosed with HIV in San Francisco. However, measuring the potential impact of these services is challenging. Building on an existing syphilis partner notification program, we developed a framework for expanding and monitoring HIV PS in San Francisco.
Methods
We identified process and outcome measures to evaluate HIV PS in San Francisco, including the number of index patients interviewed, the proportion of named partners who had previously diagnosed HIV infection, the proportion of HIV-uninfected partners who tested through HIV PS, and the positivity rate among the partners tested. Results were recorded in a locally developed electronic surveillance and case-management system at SFDPH.
Results
We examined HIV PS data from 2005–2011. In 2011, 426 new HIV diagnoses were reported, and 178 were assigned for HIV PS; of these, 124 (69.7%) patients were successfully interviewed, naming a total of 109 sex partners. Of the named partners, 34 (31.2%) had been previously diagnosed with HIV. Among the remaining named partners not known to be HIV infected, 31 (32.3%) were tested, for a positivity of 22.6% (n=7). The proportion of HIV that was newly diagnosed by a provider who participated in the citywide HIV PS program increased from 15.4% in 2005 to 69.5% in 2011.
Conclusions
As HIV PS expand, locally relevant outcome measures are increasingly important. Using these criteria, HIV PS as a targeted screening activity resulted in the identification of newly diagnosed HIV cases.
Human immunodeficiency virus (HIV) partner services (PS) encompass a broad range of services offered to people infected with HIV/acquired immunodeficiency syndrome (AIDS), including disclosing positive HIV test results; counseling about disclosing potential HIV exposure to sex and needle-sharing partners; offering third-party (health department) confidential partner notification; and making referrals to medical care, mental health, substance abuse, and other social services. In 2008, the Centers for Disease Control and Prevention (CDC) published revised guidance on PS and encouraged the coordination of these activities for HIV, syphilis, gonorrhea, and chlamydia.1 While HIV PS has been shown to be cost-effective2–4 and is well accepted by the communities served,5–7 uptake of HIV PS has been slow. A survey of U.S. health departments with the highest HIV and sexually transmitted disease (STD) morbidity in 2006 found that HIV PS was more common than in 2001, and nearly all surveyed jurisdictions provided HIV PS to those diagnosed with HIV in municipal STD clinics. However, only a minority of health departments had HIV PS activities linked to their HIV surveillance systems and, as a result, did not widely offer PS outside of health department-run sites.8
In 2010, the San Francisco Department of Public Health (SFDPH) developed its five-year HIV prevention plan, which included support for HIV PS.9 We describe the approach taken to expand HIV PS to a larger proportion of people newly diagnosed with HIV in San Francisco, including those diagnosed in private sector clinical care. The SFDPH STD Prevention and Control Services (hereafter, SFDPH STD), HIV Prevention, and HIV Epidemiology sections within SFDPH jointly implemented a program collaboration and service integration (PCSI) approach to expanding PS using a team of field and program staff, epidemiologists, and information technology staff. Additionally, this team developed locally defined measures of success, and data are provided on these baseline measures.
METHODS
Provision of HIV PS in San Francisco
HIV PS in San Francisco are performed by SFDPH STD and formally began in San Francisco in 2005, when all patients newly diagnosed with HIV at the San Francisco City Clinic (SFCC), the municipal STD clinic operated by SFDPH STD, were routinely offered PS by trained disease control investigators (DCIs) housed at the clinic. In other jurisdictions, these investigators may be known as disease intervention specialists or DIS. The DCI staff was also responsible for all syphilis partner notification, and all staff members were cross-trained to provide PS for both HIV and syphilis. HIV-positive test results were available from laboratory data reported to SFCC from the SFDPH Public Health Laboratory. A positive test result would trigger an initial interview and the offering of PS either that day (for those identified through rapid testing) or at a return visit (for those tested through standard antibody testing). All data related to the index and partners were maintained electronically in SFDPH STD's Integrated Surveillance and Clinical Health Tracking Registry (ISCHTR) system. ISCHTR is a locally developed and maintained registry system that also houses all STD surveillance case reports, the STD clinic electronic medical record, and all data related to the support of community screening and clinical sites supported by SFDPH STD.
In 2006, HIV PS were expanded beyond the STD clinic. A large public hospital, San Francisco General Hospital (SFGH), and eight SFDPH-supported primary care clinics also began to offer PS through collaboration with SFDPH STD. All newly diagnosed HIV cases from SFGH and these clinics were identified through daily data pulls from SFGH's Clinical Laboratory of HIV-positive test results reported into ISCHTR. These laboratory results were uploaded into ISCHTR, processed, and queued for HIV PS assignment to the DCI team. The same DCI staff who performed STD clinic-based HIV PS provided these services to the expanded group of clinical sites. Additionally, all new HIV-positive patients identified through SFGH were also referred to the SFGH Linkage team, which links patients with clinical and other services through the Positive Health Program, SFGH's ambulatory HIV clinic. The same interview, partner notification, and data collection and entry process used for HIV cases diagnosed at SFCC is used by SFDPH STD DCI staff for these HIV PS cases.
Beginning in 2009, HIV PS were further expanded when newly diagnosed HIV-infected patients from private provider sites in San Francisco were offered HIV PS through a process developed in collaboration with the SFDPH HIV Epidemiology Section. New reports of HIV to the HIV Epidemiology Section were checked against local and state Enhanced HIV/AIDS Reporting System registries to determine if these cases represented new or known HIV diagnoses. For HIV cases that were new diagnoses, HIV Epidemiology staff working at the provider sites reported patient contact information to the HIV PS staff and notified the diagnosing provider that HIV PS would be offered to the patient unless the provider specifically asked that the patient not be contacted. This request is usually due to mental health issues or other circumstances particular to the patient. The directors of SFDPH STD, HIV Prevention, and HIV Epidemiology sent a joint letter to all private providers in San Francisco informing them that HIV cases newly diagnosed and reported to HIV surveillance would be reported to SFDPH STD for HIV PS. The same SFDPH STD PS staff were used and the same data were collected and entered into ISCHTR.
Since the beginning of these activities, for HIV index cases diagnosed at the STD clinic, DCIs perform the initial interview and introduce HIV PS at the time of preliminary positive HIV rapid tests. People not diagnosed at the STD clinic are contacted by DCIs using all available contact information (i.e., telephone, cell phone, letter, e-mail, and field visit). Once the index case is contacted, the initial interview is conducted and partner elicitation is begun. Subsequent interviews may be conducted if necessary. In the initial interview, data collected include sociodemographic information, sexual activity and partners in the prior year, substance use in the prior year, and locations of partner recruitment. The index case is asked to provide the names and contact information for sexual and/or needle-sharing partners in the year prior to HIV diagnosis. Sex/needle-sharing partners with, at minimum, a name and either address or phone number are considered “named” partners. Partners with only an e-mail address or website handle (as used in sites such as Adam4Adam and Manhunt) are considered “Internet partners.” If, through the course of the investigation, an Internet partner is successfully contacted and additional contact information is collected (e.g., address and cell phone), that partner becomes a named partner.
DCIs attempt to contact named partners, notifying them confidentially of a possible exposure to HIV and encouraging the partner to get tested for HIV. All partners tested through the HIV PS program are offered rapid HIV testing at the STD clinic and pooled ribonucleic acid testing for detecting acute infections per clinic protocols. Additionally, screening for syphilis, chlamydia, and gonorrhea (depending on sites of exposure) are offered to partners contacted by DCI staff. Partners also have the option of testing through their own provider if they choose. Index and partner investigations are kept active for 60 days. If after 60 days they cannot be located, the case is recorded as unable to locate, with the option of reopening if new information arises.
Development of local outcome measures
As part of ongoing partnerships, SFDPH STD, HIV Prevention, and HIV Epidemiology created a multidisciplinary working group to develop locally relevant evaluation measures to monitor success in HIV PS expansion and outcomes. This group included field staff, epidemiology and data management staff, program liaisons, and management. The process and final measures were based on the recommendations outlined by CDC.1 The group identified six measures to encompass standard HIV PS evaluation: (1) number of HIV index patients interviewed (Process), (2) number and proportion of HIV index patients who named at least one partner (Process), (3) number and proportion of named partners who are HIV-positive but not newly diagnosed (longstanding positive) (Process), (4) number and proportion of HIV-uninfected named partners who tested for HIV through the SFDPH HIV PS program (Outcome), (5) HIV positivity among named partners tested through the SFDPH HIV PS program (Outcome), and (6) the proportion of newly diagnosed San Francisco HIV morbidity that was diagnosed by a provider participating in the SFDPH HIV PS program (Outcome).
Data analysis
All HIV index cases investigated by the HIV PS team from 2005 through 2011 were included in this analysis. HIV index cases who were not San Francisco residents, and therefore did not count toward San Francisco morbidity, were excluded from this analysis. Partner data were restricted to named partners and excluded any partner with only e-mail or website handle contact information. For named partners, HIV testing was restricted to any test that occurred after the date the index case was assigned for interview. This restriction removed HIV tests that could not have been a result of HIV PS activities. Data on linkage to care were not routinely collected throughout the analytic period; therefore, these data are not presented.
RESULTS
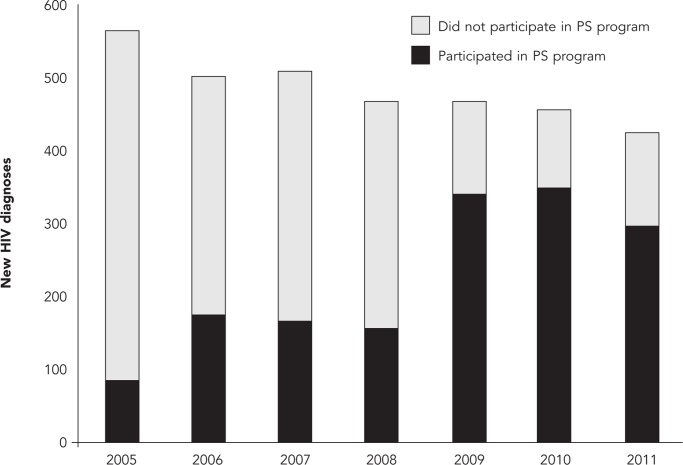
From 2005 to 2011, the number of newly diagnosed HIV infections among San Francisco residents declined 24.6% from 565 to 426 (Table). During this period, 858 (25.3% of the total new HIV diagnoses during the seven-year period) index patients participated in HIV PS (data not shown). The proportion of newly diagnosed index cases who were interviewed increased from 18.2% in 2005 to 29.1% in 2011. Furthermore, the proportion of interviewed index HIV cases who reported at least one named sex or needle-sharing partner increased from 31.1% to 40.3% during the same time period. Among the named sex and needle-sharing partners, approximately one-third were longstanding HIV-positive people (range: 13.6%–35.8%) and 27.4%–44.9% of partners not known to be HIV infected were tested as a result of HIV PS. During the seven-year analytic period, the HIV positivity among those partners tested through HIV PS was 13.2%–50.0%. The proportion of newly diagnosed HIV cases reported by a medical care provider or site that participated in the citywide HIV PS program increased from 15.4% in 2005 to 69.5% in 2011 (Table, Figure).
Table.
HIV PS outcomes for newly diagnosed HIV infections assigned for HIV PS: San Francisco, 2005–2011
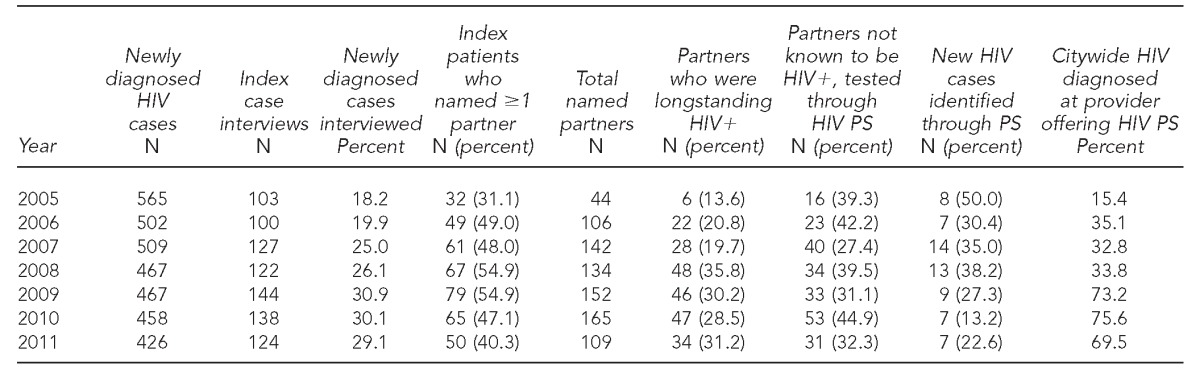
HIV = human immunodeficiency virus
PS = partner services
Figure.
Distribution of new HIV diagnoses by providers participating and not participating in the San Francisco Department of Public Health HIV Partner Services Program: San Francisco, 2005–2011
HIV = human immunodeficiency virus
PS = partner services
DISCUSSION
Expansion of HIV PS is a critical component of San Francisco's comprehensive approach to HIV prevention.9 Through collaborations across SFDPH STD, HIV Prevention, and HIV Epidemiology, the proportion of people with HIV infections diagnosed in a -clinical setting who participated in the HIV PS program increased, as did the overall proportion of index cases who participated in the HIV PS program. Bridges were created among the three sections, which resulted in more efficient use of local resources, more coordinated prevention activities, and the development of locally defined and relevant outcome measures. The success of the San Francisco program is a testament to the power of expansion of PCSI activities to improve the public's health.
HIV PS activities are cost-effective2–4 and recommended by CDC and the Community Preventive Services Task Force;1,10 however, these activities have been referred to as a missed opportunity.11 Provision of HIV PS nationally is still suboptimal.8 Structural support for health department-initiated HIV PS can increase access to these vital services. Furthermore, as shown by others,12 health department-initiated HIV PS using STD program staff who have extensive experience in partner notification is ideal. Working across existing program silos, many of the barriers reported by others have been overcome in San Francisco,13 including collaborative data sharing between HIV surveillance and STD prevention. The barriers to initial implementation were not technical in nature but resulted from a lack of clarity on policies related to data sharing, security, and confidentiality. For example, once reviewed, SFDPH STD's security and confidentiality policies were largely in alignment with those outlined in the CDC guidance;10 a new paper shredder was the only capital cost required to meet all of the defined criteria.
HIV PS is modeled after the syphilis partner notification process developed in the 1930s by then-Surgeon General Thomas Parran with the goal of disease interruption. Because syphilis is a curable condition with a relatively long incubation period, PS activities for syphilis can have a demonstrated impact on transmission and acquisition. HIV, however, is quite different. As suggested by Hogben and colleagues,13 because HIV is non-curable and has a long asymptomatic phase, the disease interruption framework may not be appropriate for HIV PS. In San Francisco, a targeted screening framework for HIV PS was developed. Because people named as sex or needle-sharing partners of an HIV-infected person have a higher risk of being HIV-infected (relative to populations of STD clinic patients or people seeking voluntary HIV testing), a paradigm was developed that couched HIV PS as an activity to identify people infected with HIV as quickly as possible and use PS as an opportunity to link positive individuals to care and services. HIV-infected people who are aware of their status have been shown to reduce their risk behaviors.14 Furthermore, HIV-infected people who are offered HIV PS have been shown to be more likely to notify partners.15 HIV-infected individuals linked to care and compliant with highly active antiretroviral therapy may be less likely to transmit HIV to partners.16,17 These factors suggest that, rather than acutely interrupting transmission, HIV PS may result in behavior change and more rapid identification of undiagnosed HIV infection, which may positively impact local epidemics. Data from San Francisco have shown that people identified as HIV-infected through HIV PS activities may not have been routinely screening for HIV, and PS facilitated an HIV diagnosis that may otherwise have been delayed.18
Furthermore, the differences between HIV and syphilis also make direct translation of standard PS protocols and dispositions from syphilis to HIV problematic. As described by others,19,20 standard HIV disposition codes are unclear and are often not consistently used. Locally, much confusion has occurred regarding differentiating CDC disposition codes (1 = previous positive, 2 = negative, 5 = new HIV-positive, and 6 = new HIV-negative). To overcome this challenge, locally relevant outcomes used to track and evaluate how HIV PS is meeting the overall goals of the San Francisco HIV prevention plan, notably expanding access to PS and demonstrating a high HIV seropositivity among those tested through HIV PS, were developed. These evaluation metrics included both process and outcome measures as well as more locally relevant outcomes that can be improved through training and supervision, such as the proportion of named partners not known to be HIV infected who test as a result of HIV PS.
Limitations
Several limitations deserve discussion. Because linkage-to-care data were not routinely and systematically collected throughout the analytic period, these data are not presented. However, linkage to care is a critical component of the HIV PS process, and future prospective evaluations will include these data, as they are now better integrated into the HIV PS process. Additionally, only partner disclosures that occurred through health department-initiated HIV PS are reported in this article; partners who may have been notified directly by the index or through provider-supported PS are not included. No information is available on the reasons why a newly diagnosed HIV case declined HIV PS; San Francisco is exploring qualitative data to help better understand individual barriers to HIV PS participation. While these data are specific to San Francisco and may not be applicable to other health jurisdictions, the approach to HIV PS, outcome measures, and the local paradigm shift described in this article are readily transferrable to other areas.
CONCLUSIONS
By developing a new paradigm that broadens the approach to HIV PS and fosters the creation of sustainable and collaborative relationships among SFDPH STD, HIV Prevention, and HIV Epidemiology sections, access to HIV PS in San Francisco was successfully increased. Based on the reports that shortening the time between HIV diagnosis and PS index interview resulted in improved HIV PS outcomes,21 and based on the success of embedded HIV PS programs in New York City,22 future plans include embedding HIV PS staff in two high-volume community-based HIV testing sites to expedite HIV PS soon after a diagnosis and bring PS to a greater proportion of local cases. Additionally, through the collaborative process, San Francisco is developing a coordinated approach to HIV prevention that incorporates HIV PS, linkage to care, and navigation for HIV-infected people who have fallen out of care. This collaboration has resulted in a win-win-win for the three health department sections: SFDPH STD received expanded resources to support HIV and syphilis PS, as well as offer STD screening to high-risk partners; HIV Prevention was able to efficiently leverage resources to expand HIV PS to more newly diagnosed HIV-infected patients; and HIV Epidemiology was able to establish processes to maximize the utility of HIV case reports. This coordinated approach to HIV prevention leverages strengths and resources from several programs and will likely positively impact the HIV epidemic in San Francisco while simultaneously improving program efficiencies and reducing duplicative efforts across health department programs.
Footnotes
This analysis was considered exempt from human subjects research considerations in accordance with the Code of Federal Regulations, Title 45, as these data were de-identified and were undergoing retrospective analysis for program evaluation and public health improvement purposes.
REFERENCES
- 1.Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and Chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–63. [PubMed] [Google Scholar]
- 2.Crawford PR, Villanti AC, Celentano DD, Colfax GN, Wolf W, Philip SS, et al. HIV partner services is a cost-effective intervention: findings from San Francisco. Paper presented at the 2012 National STD Prevention Conference; 2012 Mar 12–15; Minneapolis. [Google Scholar]
- 3.Varghese B, Peterman TA, Holtgrave DR. Cost-effectiveness of counseling and testing and partner notification: a decision analysis. AIDS. 1999;13:1745–51. doi: 10.1097/00002030-199909100-00019. [DOI] [PubMed] [Google Scholar]
- 4.Shrestha RK, Begley EB, Hutchinson AB, Sansom SL, Song B, Voorhees K, et al. Costs and effectiveness of partner counseling and referral services with rapid testing for HIV in Colorado and Louisiana, United States. Sex Transm Dis. 2009;36:637–41. doi: 10.1097/OLQ.0b013e3181a96d3d. [DOI] [PubMed] [Google Scholar]
- 5.Jones JL, Wykoff RF, Hollis SL, Longshore ST, Gamble WB, Jr, Gunn RA. Partner acceptance of health department notification of HIV exposure, South Carolina. JAMA. 1990;264:1284–6. [PubMed] [Google Scholar]
- 6.Passin WF, Kim AS, Hutchinson AB, Crepaz N, Herbst JH, Lyles CM. A systematic review of HIV partner counseling and referral services: client and provider attitudes, preferences, practices, and experiences. Sex Transm Dis. 2006;33:320–8. doi: 10.1097/01.olq.0000194597.16236.48. [DOI] [PubMed] [Google Scholar]
- 7.Golden MR, Hopkins SG, Morris M, Holmes KK, Handsfield HH. Support among persons infected with HIV for routine health department contact for HIV partner notification. J Acquir Immune Defic Syndr. 2003;32:196–202. doi: 10.1097/00126334-200302010-00012. [DOI] [PubMed] [Google Scholar]
- 8.Katz DA, Hogben M, Dooley SW, Jr, Golden MR. Increasing public health partner services for human immunodeficiency virus: results of a second national survey. Sex Transm Dis. 2010;37:469–75. doi: 10.1097/OLQ.0b013e3181e7104d. [DOI] [PubMed] [Google Scholar]
- 9.San Francisco Department of Public Health. 2010 San Francisco HIV prevention plan. San Francisco: SFDPH; 2010. [Google Scholar]
- 10.Task Force on Community Preventive Services. Recommendations to increase testing and identification of HIV-positive individuals through partner counseling and referral services. Am J Prev Med. 2007;33(Suppl 2):S88. doi: 10.1016/j.amepre.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MS, Swygard H. Human immunodeficiency virus infection and partner services: a monumental missed opportunity. Sex Transm Dis. 2010;37:476–7. doi: 10.1097/OLQ.0b013e3181e96e55. [DOI] [PubMed] [Google Scholar]
- 12.Malave MC, Shah D, Sackoff JE, Rubin S, Begier EM. Human immunodeficiency virus partner elicitation and notification in New York City: public health does it better. Sex Transm Dis. 2008;35:869–76. doi: 10.1097/OLQ.0b013e31817d2f82. [DOI] [PubMed] [Google Scholar]
- 13.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals: a systematic review. Am J Prev Med. 2007;33(2 Suppl):S89–100. doi: 10.1016/j.amepre.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 15.Golden MR, Dombrowski JC, Wood RW, Fleming M, Harrington RD. A controlled study of the effectiveness of public health HIV partner notification services. AIDS. 2009;23:133–5. doi: 10.1097/QAD.0b013e32831fb52f. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin Infect Dis. 2010;50(Suppl 3):S85–95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MS, McCauley M, Sugarman J. Establishing HIV treatment as prevention in the HIV Prevention Trials Network 052 randomized trial: an ethical odyssey. Clin Trials. 2012;9:340–7. doi: 10.1177/1740774512443594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M-J, Pipkin S, Marcus JL, Bernstein KT, Scheer S. Using HIV testing history to measure the success of HIV partner services. Sex Transm Dis. 2013;40:419–21. doi: 10.1097/OLQ.0b013e318283bfcb. [DOI] [PubMed] [Google Scholar]
- 19.Golden MR, Stekler J, Kent JB, Hughes JP, Wood RW. An evaluation of HIV partner counseling and referral services using new disposition codes. Sex Transm Dis. 2009;36:95–101. doi: 10.1097/OLQ.0b013e31818d3ddb. [DOI] [PubMed] [Google Scholar]
- 20.Katz DA, Hogben M, Dooley SW, Jr, Golden MR. An evaluation of the reliability of HIV partner notification disposition coding by disease intervention specialists in the United States. Sex Transm Dis. 2009;36:459–62. doi: 10.1097/OLQ.0b013e3181aaf14d. [DOI] [PubMed] [Google Scholar]
- 21.Marcus JL, Bernstein KT, Klausner JD. Updated outcomes of partner notification for human immunodeficiency virus, San Francisco, 2004–2008. AIDS. 2009;23:1024–6. doi: 10.1097/QAD.0b013e32832921a7. [DOI] [PubMed] [Google Scholar]
- 22.Udeagu CC, Shah D, Shepard CW, Bocour A, Guiterrez R, Begier EM. Impact of a New York City Health Department initiative to expand HIV partner services outside STD clinics. Public Health Rep. 2012;127:107–14. [PMC free article] [PubMed] [Google Scholar]