Abstract
Objective
HIV and sexually transmitted disease (STD) surveillance patterns in Arizona suggested the need for integrated data analyses to identify trends.
Methods
We compiled all HIV/AIDS cases diagnosed from 1998 to 2008 that were reported in Arizona and syphilis or gonorrhea cases diagnosed from 1998 to 2008 in Arizona. We used deterministic matching to identify individuals who were diagnosed with HIV and one or more STDs, and calculated time intervals between diagnoses.
Results
Of 23,940 people with HIV/AIDS reported from 1998 to 2008, 1,899 (2.6%) had at least one syphilis or gonorrhea diagnosis from 1998 to 2008. Approximately 85% of these cases reported male-to-male sexual contact. Among males with syphilis, HIV coinfection increased from 0.5% in 1998 to 29.1% in 2008. Among males with gonorrhea, HIV coinfection increased from 2.0% in 1998 to 3.1% in 2008. Among HIV cases diagnosed from 2004 to 2008 and reported with at least one syphilis or gonorrhea diagnosis, the majority of syphilis cases (76.1%) were diagnosed at or after HIV diagnosis, whereas a majority of gonorrhea cases (54.9%) were diagnosed prior to HIV diagnosis.
Conclusion
Use of the deterministic matching method identified increases in STD infections among HIV-infected people. The routine performance of this cross-matching method may be a useful tool in identifying these high-risk individuals so that targeted partner services and appropriate care referrals may be used in a timely fashion.
Human immunodeficiency virus (HIV) and sexually transmitted disease (STD) coinfection has received increasing attention as a public health concern in the last decade. An “epidemiological and biological synergy” between HIV/acquired immunodeficiency syndrome (AIDS) and other STDs has been observed and studied since the beginning of the HIV/AIDS epidemic.1–5 Studies have shown several facets of this syndemic relationship: people infected with STDs are more likely to become infected with HIV, and people coinfected with HIV and another STD are more likely to transmit HIV.1,2
Since 2001, primary and secondary syphilis diagnoses have been increasing nationally and in Arizona, particularly among men who have sex with men (MSM).6–8 Increases in cases of syphilis among MSM, many of whom are HIV-infected, have been characterized by high-risk sexual activities.6 Nationwide, the percentage of syphilis cases affecting MSM was 62% in 2009.6,7 In addition, MSM represent a large proportion of people living with HIV/AIDS infection in the United States and Arizona. National statistics from 2010 show that 80.9% of all new HIV diagnoses among males indicate MSM behavior and that the percentage of cases reporting MSM behavior has risen during the last decade.9
The Centers for Disease Control and Prevention (CDC) speculates that the rise in STDs among MSM may be a contributor to HIV incidence due to biological mechanisms of both ulcerative and inflammatory STDs and the increased risk of HIV transmission when STD infection is present.1,9 In a 2009 study across multiple cities, coinfection of HIV among those with a primary or secondary syphilis infection ranged from 30% to 74%, with a median of 44%.10 In Arizona, both overall HIV incidence rates and HIV incidence rates among men have decreased slightly from 2000 to 2009. However, HIV incidence rates among men aged 20–24 years increased from 22.7 per 100,000 population in 2000 to 28.7 per 100,000 population in 2009, peaking at 42.4 per 100,000 population in 2007. Additionally, 82% of these young men were identified as MSM (Unpublished data, Arizona Department of Health Services [ADHS]). In 2010, 86.7% of incident HIV/AIDS cases in Arizona occurred in males, and 68.4% of all incident HIV/AIDS cases in Arizona reported male-to-male sexual contact.11
In contrast with decreasing HIV rates, primary and secondary syphilis rates increased in Arizona, from 2.7 per 100,000 population in 2004 to 3.5 per 100,000 population in 2009. Among incident syphilis cases in Arizona's two largest counties (Maricopa and Pima), those identified as MSM increased from 61% in 2004 to 77% in 2009. Gonorrhea rates during this time declined from 74.2 per 100,000 population in 2004 to 49.3 per 100,000 population in 2009. Males had a gonorrhea rate of 53.8 per 100,000 population in 2009 compared with 44.8 per 100,000 population for females; data on gonorrhea cases among MSM were not available.8
The syndemic nature of these infections signals a need for integrated surveillance. However, there are many challenges to data sharing and analysis within public health systems, including differences in data-collection methods, variables, and systems. HIV confidentiality standards have historically been more stringent than those of other programs, resulting in limitations as to how the data could be used for public health action.12 Despite these challenges, HIV and STD surveillance patterns in Arizona suggested the need for further study to determine if STDs were occurring among individuals at risk of or previously infected with HIV. Syphilis and gonorrhea were of particular concern due to increasing rates among individuals identifying as MSM, a population at high risk of HIV infection. Performing routine cross-matching of HIV and STD data for the purposes of integrated partner services was identified as a public health priority. Therefore, beginning in March 2009, epidemiologists in the HIV/AIDS and STD sections of ADHS collaborated to identify people with multiple infections.
MATERIALS AND METHODS
We compiled all records of HIV or AIDS cases diagnosed from 1998 to 2008 in the Arizona Enhanced HIV/AIDS Reporting System (eHARS). This analysis included both cases initially diagnosed in Arizona and cases initially diagnosed in other jurisdictions who later resided in Arizona. Records of all syphilis and gonorrhea events diagnosed in Arizona from 1998 through 2008 were abstracted from the state STD surveillance database. Arizona has legislation pertaining to both HIV and STD reporting requirements.13 ADHS maintains HIV surveillance data in eHARS, a browser-based application designed for HIV/AIDS reporting. STD data are maintained in an Oracle-based system (NAT-P). Both databases include an intersection of laboratory and provider reports along with data collected during partner services interviews.
We abstracted demographic information (e.g., sex, date of birth, and race/ethnicity) from both databases and calculated age using the date of the first disease event. All risk behavior information came from the HIV dataset except for sexual preference, which was present in both datasets. For the small number of cases in which the demographic or sexual preference data differed between the two datasets, data from the HIV dataset were used. We used deterministic matching using the first and third characters of the first name, first and third characters of the last name, date of birth, and gender to identify individuals who had been reported with multiple diseases.14 Pairs that exactly matched on all data elements were considered to be matches. Pairs were classified as potential matches if some data elements matched. Potential matches underwent manual review, and reviewers coded each pair as a match or a nonmatch; in cases in which a decision was uncertain, reviewers were instructed to classify the match as false. These data were then de-identified, and links to the original datasets were removed. Cases with both syphilis and gonorrhea diagnoses and no HIV infection were excluded from our analyses. Additionally, analyses were stratified by gender; people with a gender other than male or female were excluded from all gender-stratified analyses.
Differences in demographic information between people with HIV only and people with multiple infections were assessed using Chi-square testing. For people with HIV infection, we determined the number who had syphilis diagnosed before, after, or at the same time of HIV diagnosis. Similarly, for people with HIV infection, we determined the number who had gonorrhea diagnosed before, after, or at the same time as their HIV diagnosis. People with multiple STD diagnoses were counted once, using the data from the most recent STD diagnosis. The percentage of people with one or more syphilis and gonorrhea diagnoses and previous HIV infection was also calculated by year of first STD diagnosis. STD cases were considered previously infected with HIV if there was a syphilis or gonorrhea diagnosis more than 60 days after HIV diagnosis. Concurrent infections were defined as those that were diagnosed within 60 days of HIV diagnosis.
To assess more recent trends in disease diagnosis, we calculated time intervals between disease events for people with an HIV diagnosis from 2004–2008 and a previous, concurrent, or subsequent STD diagnosis. We calculated time intervals in months for each individual by subtracting the date of HIV diagnosis from the most recent date of syphilis or gonorrhea diagnosis. People were considered to be diagnosed with syphilis or gonorrhea after HIV diagnosis if the STD diagnosis was more than two months after HIV diagnosis. All analyses were completed using SAS® version 9.2.15
RESULTS
There were 72,480 people with one or more diagnoses including HIV (n=23,940), syphilis (n=10,102 diagnoses among 9,680 people), and gonorrhea (n=46,953 diagnoses among 40,969 people). Of the 23,940 people reported with HIV, 1,899 (2.6%) had syphilis or gonorrhea diagnosed before, after, or at the same time as their HIV infection (Table).
Table.
Demographic characteristics of HIV and STD cases diagnosed from 1998–2008 and reported in Arizona
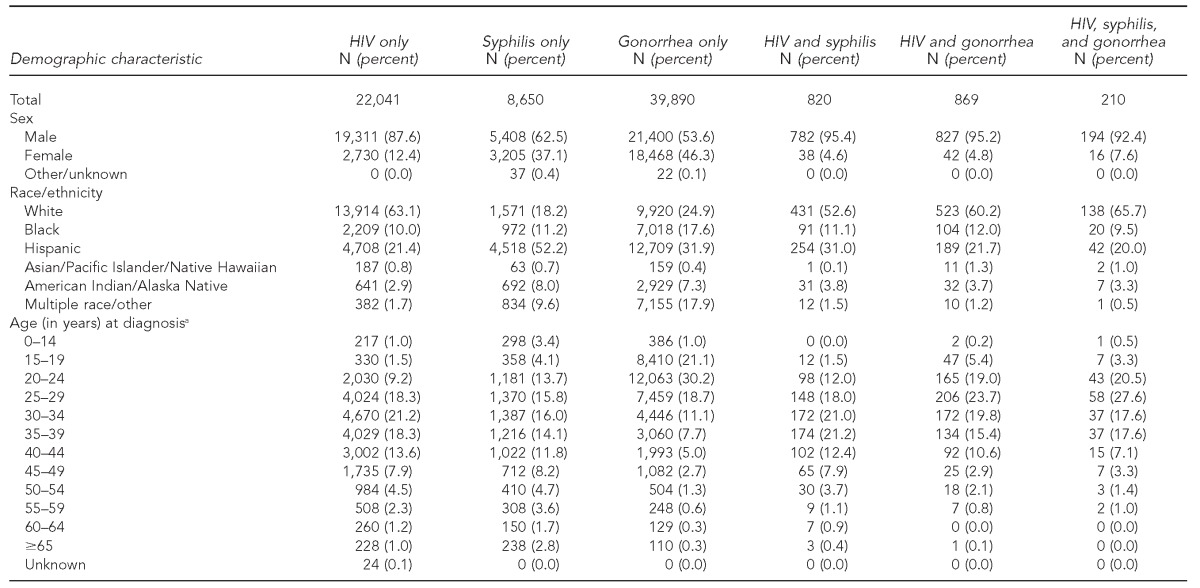
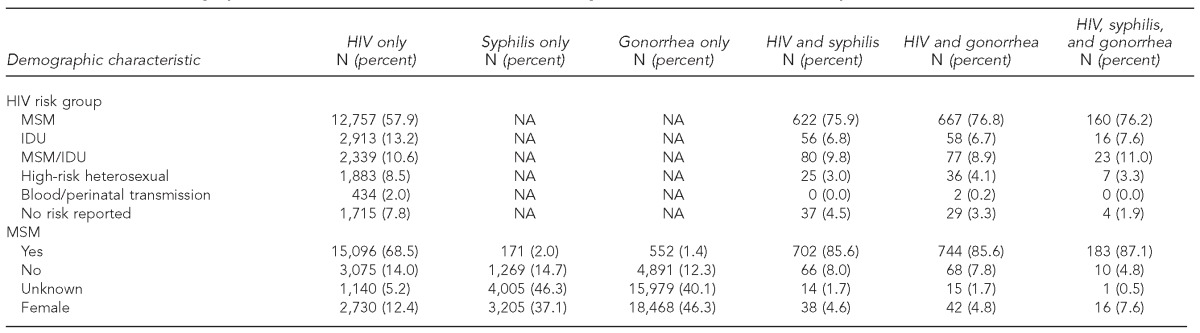
aFor cases with multiple diagnoses, age at first disease event
HIV = human immunodeficiency virus
STD = sexually transmitted disease
MSM = men who have sex with men
NA = not applicable
IDU = injection drug user
Compared with people with HIV only, people with multiple infections were, on average, more likely to be male (94.9% vs. 87.6%, Chi-square = 90.25, p<0.0001). Compared with people who had HIV only, people with multiple infections were also, on average, more likely to be Hispanic (25.5% vs. 21.4%, Chi-square = 17.98, p<0.0001) and aged 20–29 years at first disease event (37.8% vs. 27.5%, Chi-square = 92.19, p<0.0001). Male-to-male sexual contact was reported more often, on average, for people with multiple infections (85.8%) than for people with HIV only (68.5%, Chi-square = 248.28, p<0.0001) (data not shown).
Among people diagnosed with HIV/AIDS by the end of 2008 and reported in Arizona, 4.3% (n=1,030) had a previously (n=173), concurrently (n=185), or subsequently (n=672) diagnosed syphilis infection (at any stage) in Arizona. Of these 1,030 cases, 94.8% (n=976) were among males. The rate of a previous, concurrent, or subsequent syphilis diagnosis among men diagnosed with HIV was 4,632 per 100,000 population, while the rate of syphilis diagnosis among the general male population was 246 per 100,000 population.
Similar results were seen with gonorrhea. Among people with HIV/AIDS, 4.5% (n=1,079) had a previously (n=386), concurrently (n=86), or subsequently (n=607) reported gonorrhea infection in Arizona. Of these 1,079 cases, 94.6% (n=1,021) were male. The rate of a previous, concurrent, or subsequent gonorrhea infection report among men diagnosed with HIV was 4,836 per 100,000 population compared with a rate of gonorrhea infection among the general male population of 815 per 100,000 population (data not shown).
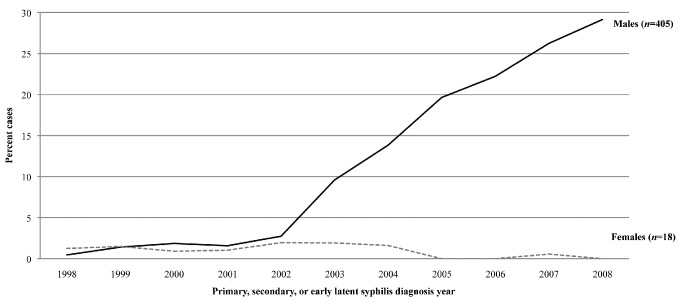
Over time, an increasing percentage of males with a history of both HIV and syphilis and/or gonorrhea infection was observed. This analysis showed a sharp increase in the percentage of males with incident syphilis who were previously infected with HIV (defined as being diagnosed with HIV more than 60 days prior to the syphilis diagnosis) starting in 2003. This percentage increased from 0.5% in 1998 to 29.1% in 2008 (Figure 1). The majority of these coinfected males reported male-to-male sexual contact; of the 405 males diagnosed with HIV prior to their early syphilis diagnosis, 384 (94.8%) reported male-to-male sexual contact (data not shown). Again, similar results were seen with gonorrhea, though the increase was more modest. The percentage of males with incident gonorrhea who were previously infected with HIV increased from 2.0% in 1998 to 3.5% in 2007 (Figure 2). The majority of these coinfected males also reported male-to-male sexual contact. Of the 581 males diagnosed with HIV prior to their gonorrhea diagnosis reported from 1998–2008, 541 (93.1%) reported male-to-male sexual contact (data not shown).
Figure 1.
Percentage of people with syphilis who were coinfected with HIV, by sex: Arizona, 1998–2008
HIV = human immunodeficiency virus
Figure 2.
Percentage of people with gonorrhea who were coinfected with HIV, by sex: Arizona, 1998–2008
HIV = human immunodeficiency virus
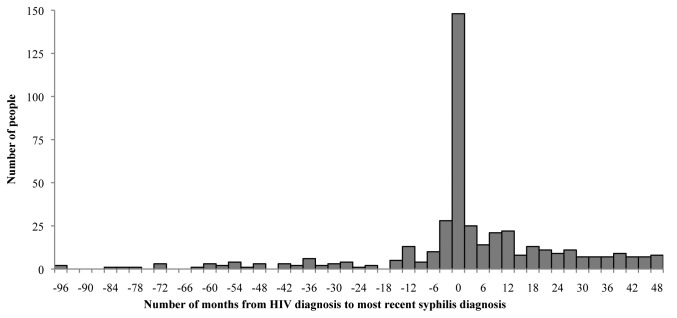
Among the 439 HIV cases diagnosed from 2004–2008 that had a reported syphilis history, 76.1% (n=334 of 439) of people diagnosed with syphilis were diagnosed after (n=186 of 439, 42.4%) or at the time of (n=148 of 439, 33.7%) HIV diagnosis, while 23.9% (n=105 of 439) were diagnosed before HIV diagnosis (Figure 3). Among cases diagnosed with syphilis after HIV diagnosis, the median time from HIV diagnosis to the most recent syphilis diagnosis was 18.0 months, with a mean of 21.2 months (standard deviation [SD] = 14.3). The results were similar when earliest syphilis diagnosis was used (data not shown).
Figure 3.
Distribution of time lapse in months between earliest HIV diagnosis and most recent syphilis diagnosis among HIV/syphilis comorbid people: Arizona, 2004–2008 (n=439)
HIV = human immunodeficiency virus
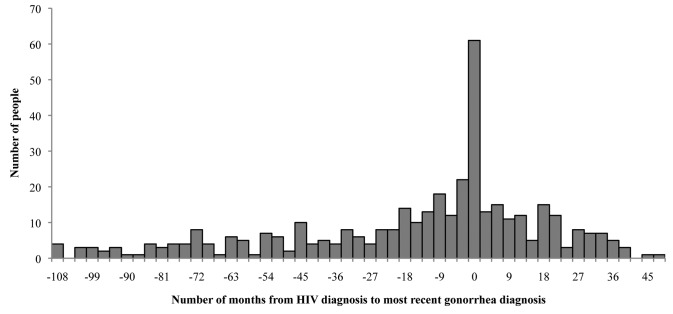
HIV cases with a reported gonorrhea history showed a contrasting time-interval pattern compared with HIV cases with a reported syphilis history. Among the 397 HIV cases with a reported gonorrhea history diagnosed with HIV from 2004–2008, 54.9% of people diagnosed with gonorrhea (n=218 of 397) were diagnosed before HIV diagnosis. In this population, only 15.4% (n=61 of 297) of HIV cases diagnosed from 2004–2008 were diagnosed with gonorrhea at HIV diagnosis, and 29.7% (n=118 of 297) of HIV cases were diagnosed with gonorrhea after HIV diagnosis (Figure 4). Among cases diagnosed with gonorrhea before HIV diagnosis, the median time from the most recent gonorrhea diagnosis to HIV diagnosis was 35.9 months, with a mean of 27.5 months (SD=30.3). The results were similar when the earliest gonorrhea diagnosis was used. During this time period, the rate of a previous, concurrent, or subsequent HIV infection reported in people diagnosed with gonorrhea was 1,965 per 100,000 population compared with a general rate of HIV infection of 14 per 100,000 population (data not shown).
Figure 4.
Distribution of time lapse in months between earliest HIV diagnosis and most recent gonorrhea diagnosis among HIV/gonorrhea coinfected people: Arizona, 2004–2008 (n=397)
HIV = human immunodeficiency virus
DISCUSSION
We observed an increase in the number of HIV-infected males with a subsequent syphilis or gonorrhea infection in Arizona. Of these coinfected males, more than 90% reported male-to-male sexual contact. During 1998–2008, the majority of cases with multiple diagnoses were diagnosed with syphilis or gonorrhea at or after HIV diagnosis. Among people diagnosed with HIV in 2004–2008, the majority of gonorrhea diagnoses occurred before the HIV diagnosis, highlighting this infection as a possible sentinel event for HIV diagnosis. Overall Arizona HIV and gonorrhea rates have decreased, while rates of syphilis have increased. Nationally, a 2009 study found that 36% of individuals diagnosed with syphilis were also coinfected with HIV. Among MSM diagnosed with syphilis, 53% reported HIV infection.10 Our study showed similar findings.
This analysis demonstrates that some people diagnosed with HIV, syphilis, or gonorrhea continue to engage in high-risk sexual practices after diagnosis. As a result, these patients are contracting additional infections and exposing their partners to HIV and/or acute STD infection. The results suggest that male-to-male sexual contact is a risk factor for multiple infections. During the study time period, the annual number of syphilis cases increased, as did the number of HIV cases among young men, but there was no corresponding increase in overall HIV rates. Possible explanations for an observed increase in HIV-coinfected syphilis and gonorrhea cases without coincident increases in HIV are serosorting and the use of highly active antiretroviral therapy (HAART). Serosorting, the practice of seeking out sexual partners of the same HIV status, is a common practice among MSM.16,17 One study estimated that 21%–62% of the MSM population participated in this practice.18 The use of HAART has been associated with reduced HIV transmission in serodiscordant partners, and reduced genital viral loads may dampen the risk of HIV acquisition and/or transmission among MSM with a concurrent STD.19,20 Although serosorting and the use of HAART may account for some of these findings, collection of this information was beyond the scope of this analysis.
Similar to other jurisdictions, there were numerous reported cases of STDs at or after HIV diagnosis in Arizona.21–26 The public health implications of HIV/syphilis and HIV/gonorrhea coinfection are significant. Coinfection suggests that people infected with HIV are continuing to engage in high-risk sexual behavior whether or not their partners are of the same HIV serostatus. This analysis also shows that many gonorrhea diagnoses are occurring before HIV diagnosis, indicating that people with gonorrhea may be at higher risk of HIV infection at a later date. It is possible that our results are due to a lack of HIV testing among people with gonorrhea. The symptoms of gonorrhea appear more quickly than the symptoms of HIV (and syphilis), and a nationwide study estimated that only 51% of gonorrhea patients had been tested for HIV at gonorrhea diagnosis.27 However, our results still suggest that gonorrhea infection indicates a high current or future risk of HIV infection.
Identifying coinfected individuals makes it possible to perform integrated partner services and develop prevention efforts targeting both STDs and HIV.28 For example, knowledge of positive HIV status of syphilis and gonorrhea cases should prompt more aggressive referral of sexual partners for HIV testing. Arizona lacks the public health resources to offer partner services to every person diagnosed with syphilis or gonorrhea, and prioritizing cases with a previous HIV infection is a possible tool for identifying undiagnosed HIV cases. In addition, identifying high-risk HIV cases with STDs that have fallen out of primary HIV medical care may provide opportunities for renewed linkage to care. Performing timely comorbidity analyses of HIV and STD surveillance data is a necessary step in facilitating these public health interventions.
Limitations
This study was subject to several limitations. Underreporting is inherent in passive surveillance systems. Due to the asymptomatic nature of HIV, many people are unaware of their infection(s) and, thus, remain undiagnosed. Additionally, this study used HIV prevalence measures rather than incidence measures, and the exact time sequence of the infections cannot be determined. It is also possible that people in HIV care are more likely to be tested for other STDs, and that increased awareness of HIV among MSM resulted in higher testing rates of both HIV and other STDs. It is also important to note that HIV prevalence in Arizona increased 30% from 2006 to 2011, and the number of deaths among those infected with HIV decreased during that same time period.11 Longer life spans among HIV-infected people may have led to an increase in the number of people acquiring an STD after infection in our sample. The databases used in this study did not contain detailed risk factor information, so it was not possible to determine which specific behaviors resulted in coinfection and are potential targets for intervention.
Inconsistencies in the format and completeness of the data among the two databases, as well as reviewer error, likely reduced the accuracy of the deterministic matching process, which is dependent on the data entered in a small number of fields. Additionally, reliance solely on deterministic matching is not recommended for use at the patient level.14 Before data can be used for targeted partner services, time-consuming manual review of complete patient records will be necessary to ensure accuracy.
CONCLUSIONS
Confidential partner services is a public health tool whereby partners of infected cases are informed of their STD/HIV exposure so that the partner can receive testing and treatment.28 Integrated data surveillance is critical to guide partner services among those who are coinfected. In Arizona, syphilis cases are prioritized over gonorrhea cases for investigation due to the increased morbidity associated with syphilis and the large number of gonorrhea cases each year. Prompt identification of coinfected individuals through an integrated surveillance system can allow for timely and prioritized partner services through which these high-risk populations can be educated about the prevention of disease transmission and receive information about testing and treatment efforts. ADHS HIV and STD programs have collaborated to institute such a surveillance system to identify coinfected cases, which allows for the most targeted partner services response. We hope that interventions at the client level will facilitate the identification of undiagnosed HIV cases and provide linkages to care, and that increased partner services and treatment efforts will decrease HIV and STD transmission in Arizona. Future studies to determine the effectiveness of this real-time surveillance system are needed to determine its efficacy.
Footnotes
The authors thank Rick DeStephens, Arizona Department of Health Services HIV Surveillance Program Manager, for his guidance and editorial contribution. This article was presented in part as an abstract at the International Society for STD Research Conference in Quebec City, Canada, in July 2011.
The analysis of these data was for surveillance purposes and did not involve research or human subjects. The protocol was reviewed under the U.S. Department of Health and Human Services Office of Human Research Protection guidelines and determined to not be subject to Institutional Review Board review. This determination was approved by the Arizona Department of Health Services (ADHS) management.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of ADHS, the Centers for Disease Control and Prevention, or the Maricopa County Department of Public Health.
REFERENCES
- 1.HIV prevention through early detection and treatment of other sexually transmitted diseases—United States. Recommendations of the Advisory Committee for HIV and STD Prevention. MMWR Recomm Rep. 1998;47(RR-12):1–24. [PubMed] [Google Scholar]
- 2.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and Chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537–43. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 4.Jin F, Prestage GP, Imrie J, Kippax SC, Donovan B, Templeton DJ, et al. Anal sexually transmitted infections and risk of HIV infection in homosexual men. J Acquir Immune Defic Syndr. 2010;53:144–9. doi: 10.1097/QAI.0b013e3181b48f33. [DOI] [PubMed] [Google Scholar]
- 5.Huhn GD, McIntyre AF, Broad JM, Holmes SW, Studzinski A, Rabins C, et al. Factors associated with newly diagnosed HIV among persons with concomitant sexually transmitted diseases. Sex Transm Dis. 2008;35:731–7. doi: 10.1097/OLQ.0b013e31817f97a0. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (US), Division of STD Prevention. Sexually transmitted disease surveillance 2009. Atlanta: CDC; 2010. Also available from: URL: http://www.cdc.gov/std/stats09/surv2009-Complete.pdf [cited 2011 Jul 13] [Google Scholar]
- 7.Heffelfinger JD, Swint EB, Berman SM, Weinstock HS. Trends in primary and secondary syphilis among men who have sex with men in the United States. Am J Public Health. 2007;97:1076–83. doi: 10.2105/AJPH.2005.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arizona Department of Health Services. Sexually transmitted diseases in Arizona: 2009 annual report. Phoenix (AZ): ADHS; 2010. Also available from: URL: http://azdhs.gov/phs/oids/std/pdf/STD%20Annual%20Report%202009%20.pdf [cited 2011 Nov 15] [Google Scholar]
- 9.Centers for Disease Control and Prevention (US) HIV surveillance in men who have sex with men (MSM) [cited 2011 Aug 19.] Available from: URL: http://www.cdc.gov/hiv/topics/surveillance/resources/slides/msm/index.htm.
- 10.Su JR, Weinstock HS. Epidemiology of co-infection with HIV and syphilis in 34 states, United States, 2009. Abstract presented at the National HIV Prevention Conference; 2011 Aug 14–17; Atlanta. [Google Scholar]
- 11.Arizona Department of Health Services. HIV epidemiology program: 2011 annual report [cited 2012 Apr 2] Available from: URL: http://azdhs.gov/phs/hiv/reporting/2011report.htm.
- 12.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2011. Data security and confidentiality guidelines for HIV, viral hepatitis, sexually transmitted disease, and tuberculosis programs: standards to facilitate sharing and use of surveillance data for public health action. [Google Scholar]
- 13.Arizona Adm. Code. R9-6-202 [cited 2011 Dec 1] Available from: URL: http://www.azsos.gov/public_services/Table_of_Contents.htm.
- 14.Newman LM, Samuel MC, Stenger MR, Gerber TM, Macomber K, Stover JA, et al. Practical considerations for matching STD and HIV surveillance data with data from other sources. Public Health Rep. 2009;124(Suppl 2):7–17. doi: 10.1177/00333549091240S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SAS Institute, Inc. Cary (NC): SAS Institute, Inc.; 2009. SAS®: Version 9.2 for Windows. [Google Scholar]
- 16.Truong HM, Kellogg T, Klausner JD, Katz MH, Dilley J, Snapper K, et al. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex Transm Infect. 2006;82:461–6. doi: 10.1136/sti.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008;49:212–8. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 18.Eaton LA, Kalichman SC, O'Connell DA, Karchner WD. A strategy for selecting sexual partners believed to pose little/no risks for HIV: serosorting and its implications for HIV transmission. AIDS Care. 2009;21:1279–88. doi: 10.1080/09540120902803208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley CF, Haaland RE, Patel P, Evans-Strickfaden T, Farshy C, Hanson D, et al. HIV-1 RNA rectal shedding is reduced in men with low plasma HIV-1 RNA viral loads and is not enhanced by sexually transmitted bacterial infections of the rectum. J Infect Dis. 2011;204:761–7. doi: 10.1093/infdis/jir400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hague JC, Muvva R, Miazad RM. STD coinfection and reinfection following HIV diagnosis: evidence of continued sexual risk behavior. Sex Transm Dis. 2011;38:347–8. doi: 10.1097/OLQ.0b013e3181fc6ace. [DOI] [PubMed] [Google Scholar]
- 22.Brewer TH, Schillinger J, Lewis FM, Blank S, Pathela P, Jordahi L, et al. Infectious syphilis among adolescent and young adult men: implications for human immunodeficiency virus transmission and public health interventions. Sex Transm Dis. 2011;38:367–71. doi: 10.1097/OLQ.0b013e3181ffa7b0. [DOI] [PubMed] [Google Scholar]
- 23.Mayer KH, Bush T, Henry K, Overton ET, Hammer J, Richardson J, et al. Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions. Sex Transm Dis. 2012;39:1–7. doi: 10.1097/OLQ.0b013e31823b1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaulding AB, Lifson AR, Iverson ER, Ganesan A, Landrum ML, Weintrob AC, et al. Gonorrhoea or Chlamydia in a U.S. military HIV-positive cohort. Sex Transm Infect. 2012;88:266–71. doi: 10.1136/sextrans-2011-050173. [DOI] [PubMed] [Google Scholar]
- 25.Stenger MR, Courogen MT, Carr JB. Trends in Neisseria gonorrhoeae incidence among HIV-negative and HIV-positive men in Washington State, 1996–2007. Public Health Rep. 2009;124(Suppl 2):18–23. doi: 10.1177/00333549091240S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen M. STD after HIV diagnosis. Poster presentation at the 2012 National STD Prevention Conference; 2012 Mar 12–15; Minneapolis. [Google Scholar]
- 27.Bradley H, Asbel L, Bernstein K, Mattson M, Pathela P, Mohamed M, et al. HIV testing among patients infected with Neisseria gonorrhoeae: STD Surveillance Network, United States, 2009–2010. AIDS Behav. 2013;17:1205–10. doi: 10.1007/s10461-012-0304-0. [DOI] [PubMed] [Google Scholar]
- 28.Dooley SW. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and Chlamydial infection. MMWR Recomm Rep. 2008;57(RR-09):1–63. [PubMed] [Google Scholar]