Abstract
Objective
Critical congenital heart disease (CCHD) was recently added to the U.S. Recommended Uniform Screening Panel for newborns. This evaluation aimed to estimate screening time and hospital cost per newborn screened for CCHD using pulse oximetry as part of a public health economic assessment of CCHD screening.
Methods
A cost survey and time and motion study were conducted in well-newborn and special/intensive care nurseries in a random sample of seven birthing hospitals in New Jersey, where the state legislature mandated CCHD screening in 2011. The sample was stratified by hospital facility level, hospital birth census, and geographic location. At the time of the evaluation, all hospitals had conducted CCHD screening for at least four months.
Results
Mean screening time per newborn was 9.1 (standard deviation = 3.4) minutes. Hospitals' total mean estimated cost per newborn screened was $14.19 (in 2011 U.S. dollars), consisting of $7.36 in labor costs and $6.83 in equipment and supply costs.
Conclusions
This federal agency-state health department collaborative assessment is the first state-level analysis of time and hospital costs for CCHD screening using pulse oximetry conducted in the U.S. Hospitals' cost per newborn screened for CCHD with pulse oximetry is comparable with cost estimates of existing newborn screening tests. Hospitals' equipment costs varied substantially based on the pulse oximetry technology employed, with lower costs among hospitals that used reusable screening sensors. In combination with estimates of screening accuracy, effectiveness, and avoided costs, information from this evaluation suggests that CCHD screening is cost-effective.
In September 2011, the U.S. Secretary of Health and Human Services approved the addition of critical congenital heart disease (CCHD) to the Recommended Uniform Screening Panel for newborns.1 Just before that approval, New Jersey became the first state to implement mandatory pulse oximetry screening in all licensed birthing facilities to improve detection and early intervention for newborns with CCHD.2 Similar legislation has since been introduced or enacted in many other states. Though clinical evidence supports routine CCHD screening,3,4 at least one earlier attempt at passing state legislation was stymied by reservations that included cost concerns.5
Congenital heart disease affects an estimated nine per 1,000 live births in the United States; approximately one-quarter of those children have critical conditions requiring surgery or catheter intervention during infancy.3,6 Newborns with untreated CCHD are at risk for cardiovascular collapse within the first days of life,3 although some newborns do not present obvious physical signs of their condition before birth hospital discharge. Newborns with CCHD not detected during prenatal screening or postnatal examinations may benefit from routine screening at birth hospitals.
Previous estimates of screening time and cost
Estimates of the time and cost of pulse oximetry screening have appeared in the recent literature. Two studies—both from the United Kingdom—reported observations of the time spent by staff engaged in the screening process. One study reported a mean screening time of 2.0 minutes per newborn, where screening included one pulse oximetry reading conducted by a doctor during a clinical examination.7,8 The second study reported a mean screening time per newborn of 6.9 minutes, based on a survey completed by midwives who conducted the screening.9,10 The two studies reported an estimated cost per newborn screened—including labor and equipment—of $6.13 and $9.97, respectively (both estimates expressed as 2011 U.S. dollars11,12). Recent U.S. studies provided estimates of mean screening times per newborn ranging from 45 seconds to 3.5 minutes (where screening usually included one pulse oximetry reading), and estimates of equipment-only screening costs per newborn ranging from “negligible” to $11.00.3,5,6,13–15 No previous estimates were based on reported systematic studies in which observers objectively recorded screening times.
Robust estimates of hospitals' costs to conduct routine newborn screening for CCHD using pulse oximetry may inform hospitals' and states' decisions on such screening. We aimed to estimate the screening time and cost—including labor and equipment—for hospitals to screen newborns for CCHD using pulse oximetry. This information was collected as part of a broader public health economic assessment of CCHD screening, which is summarized hereafter.
METHODS
This evaluation was a collaboration between the Centers for Disease Control and Prevention (CDC) and the New Jersey Department of Health (NJDOH). This evaluation included a cost survey of hospital administrators and a time and motion study of CCHD screening undertaken by two CDC staff in well-newborn and special/intensive care nurseries in a sample of New Jersey hospitals.
New Jersey screening protocol
NJDOH's screening protocol recommends newborns be screened for CCHD through pulse oximetry 24–48 hours after birth or shortly before discharge if discharged in <24 hours. Infants requiring special/intensive care may be screened at discharge, which can exceed the neonatal period (<28 days). The NJDOH-recommended protocol includes both pre- and post-ductal pulse oximetry measurements (from the hand and foot, respectively) for each newborn. Nursery staff may perform up to three screens per newborn if an initial screening does not indicate sufficient blood oxygen saturation in the newborn.
Hospital sample
The New Jersey legislative mandate for all birthing facilities to screen newborns for CCHD provided an opportunity for the systematic collection of information from a random sample of hospitals to estimate screening time and cost. The sampling methodology used to select hospitals was designed to be representative of New Jersey birthing hospitals. Of the 52 licensed birthing facilities in New Jersey in 2010 (most recent available information), 42 had facility Levels 3–5 (Level 5 is the state's highest designation for critical care), which accounted for 96% of New Jersey hospital births. A random sample of seven hospitals (or 17% of New Jersey Level 3–5 birthing facilities), consisting of two each of Levels 3 and 4 and three of Level 5, was stratified by hospital birth census, regional location (based on administrative boundaries used by NJDOH), and county. Hospitals' birth counts are not reported in this article to protect participating hospitals' anonymity. This evaluation took place from January to February 2012, which was approximately four months after hospitals were required to implement routine CCHD screening.
Time and motion study
Two of the authors timed a minimum of three screenings with stopwatches at each hospital during visits planned in advance with hospital administrators. All timings were initiated/concluded when hospital staff began/ended activities related exclusively to screening. For example, five out of seven hospitals conducted screening once newborns were assembled in the nursery for other routine screenings. In such circumstances, transit time to bring the newborn to the nursery or return the newborn to the mother's room was not included. All timings ended when staff had disposed of or cleaned the equipment and completed documentation. Depending on the hospital, such documentation may have included adding the screening results to the newborn's electronic or paper medical record and completing documentation for pediatricians and parents. If no newborns were scheduled for CCHD screening, researchers watched nursery staff perform simulated screens with newborns who had already undergone screening or were not yet 24 hours of age. In such cases, all procedures and documentation were carried out as usual, with the exception that screening results were not entered into the hospital's final documentation.
Cost survey
A cost survey was distributed to hospital administrators. The survey requested information on the level of staff (such as registered nurse [RN]) who performed CCHD screening, the mean salary for those staff, and the hospital's fringe benefit percentage. Hospital administrators were also asked about pulse oximetry machine purchase prices, the useful clinical life of the machines, costs for annual machine maintenance (including labor and replacement parts), and the cost of disposable components, including sensors (sometimes called probes) and, for some sensors, wraps to hold the sensors in place on a newborn's hand or foot. All reported costs were converted to 2011 values.12 Fixed equipment costs were calculated as the sum of amortized machine purchase costs—based on an average reported clinical life of seven years—and annual replacement parts and labor. Overall per-newborn measures, regardless of nursery facility, were calculated as weighted averages using a national estimate that 6.7% of newborns are admitted to special/intensive care nurseries.16
RESULTS
Screening time
A total of 23 newborn pulse oximetry screenings were observed in seven New Jersey hospitals. No newborns had abnormal results or needed to be rescreened. The mean screening time in well-newborn nurseries for all observations was higher than in special/intensive care nurseries (9.1 minutes, standard deviation [SD] = 3.8, range: 4.0–16.4 [n=15] vs. 6.9 minutes, SD=1.9, range: 3.8–9.5 [n=8]) (Table 1). Among 11 routine screens, eight were in well-newborn nurseries, and three were in special/intensive care nurseries, with a mean screening time of 10.2 (SD=4.1, range: 5.8–16.4) and 8.2 (SD=1.2, range: 7.2–9.5) minutes, respectively (data not shown). Among 12 simulated screens, seven were in well-newborn nurseries, and five were in special/intensive care nurseries, with a mean screening time of 7.9 (SD=3.2, range: 4.0–12.1) and 6.1 (SD=1.8, range: 3.8–8.4) minutes, respectively (data not shown). The weighted average screening time per newborn, regardless of nursery facility, was 9.1 (SD=3.7) minutes (Table 1).
Table 1.
Observed CCHD screening time at seven New Jersey hospitals and estimated hospital labor costs for well-newborn nurseries and special/intensive care nurseries: January–February 2012
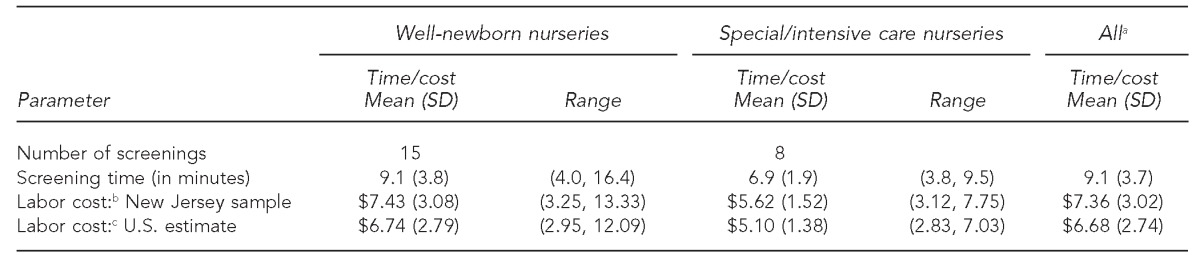
Notes: All cost estimates are presented as 2011 values based on: Bureau of Labor Statistics (US). Producer price index industry data: hospitals. PCU622 2011 [cited 2012 May 18]. Available from: URL: http://www.bls.gov/data/#prices. Screenings observed from January to February 2012. Numbers may not sum precisely due to rounding.
aWeighted average and SD of screening times from well-newborn and special/intensive care nurseries were based on a national estimate that 6.7% of newborns are admitted to special/intensive care nurseries. Source: Osterman MJ, Martin JA, Mathews TJ, Hamilton BE. Expanded data from the new birth certificate, 2008. Natl Vital Stat Rep 2011 Jul 27;59(7):1-28.
bBased on an estimated hourly labor cost from the New Jersey hospital survey results of $48.81 (including fringe benefits). The range represents the hourly cost applied to high and low observed screening times.
cBased on 2011 national average annual wage ($69,110) for registered nurses and the fringe benefit percentage reported in cost surveys collected in this evaluation (33.2%), resulting in an estimated hourly wage of $44.26. Source: Bureau of Labor Statistics (US). Occupational employment and wages, May 2011: 29-1111 registered nurses. 2011 [cited 2012 May 7]. Available from: URL: http://www.sbls.gov/oes/current/oes291111.htm#(2)
CCHD = critical congenital heart disease
SD = standard deviation
The number of observed screenings was too small to permit a meaningful statistical analysis of the apparent difference in mean screening time in the well-newborn and special/intensive care nurseries. However, all of the special/intensive care nurseries already used continuous oxygen saturation monitoring on newborns. For CCHD screening, staff in special/intensive care nurseries noted the oxygen saturation level from the newborn's extremity displayed on a vital signs monitor and obtained the second required reading either by moving the sensor temporarily to the other extremity or by using another sensor and machine.
Labor costs
All hospitals reported that RNs performed CCHD screening. The mean reported salary for RNs was $76,204 (SD=$5,386, range: $67,931–$83,000), with an additional fringe benefit of 33.2% (SD=6.1%) of salary. Assuming a 40-hour workweek (2,080 work hours per year), this mean salary and fringe benefit was equal to an estimated hourly labor cost of $48.81 (SD=$3.97) (data not shown). By applying this hourly labor cost to the observed screening times, hospitals' estimated mean labor cost per newborn screened was $7.43 (SD=$3.08, range with minimum and maximum observed screening times: $3.25–$13.33) in well-newborn nurseries and $5.62 (SD=$1.52, range: $3.12–$7.75) in special/intensive care nurseries (Table 1). Using the 2011 national estimate for an RN annual salary ($69,110) from the U.S. Bureau of Labor Statistics, the estimated labor cost of CCHD screening nationwide was $6.74 (SD=$2.79, range: $2.95–$12.09) in well-newborn nurseries and $5.10 (SD=$1.38, range: $2.83–$7.03) in special/intensive care nurseries (Table 1).17 The weighted average screening cost per newborn, regardless of nursery facility, was $7.36 (SD=$3.02) in New Jersey and $6.68 (SD=$2.74) in the U.S. (Table 1).
Pulse oximetry screening sensors and cost
Well-newborn nurseries.
The seven hospitals used three variations of pulse oximetry sensor technology in well-newborn nurseries: fully disposable sensors (n=3 hospitals), partially reusable sensors (n=3 hospitals), and fully reusable sensors (n=1 hospital) (Table 2). The partially reusable sensors included a disposable wrap to hold a reusable sensor in place on the newborn's hand or foot; the reusable sensor was wiped down with alcohol after each screening. The fully reusable sensor was held in place with a rubber device cinched around the newborn's hand or foot. Hospitals' mean costs for disposable screening components per newborn ranged from zero dollars at the hospital that used fully reusable sensors to $16.44 at one hospital that used two disposable sensors (one sensor for each hand and foot reading) per well-newborn. The cost of cleaning supplies (alcohol and swabs) for reusable sensors was assumed to be negligible.
Table 2.
Estimateda hospital equipment costs per newborn screened for CCHD at seven New Jersey based on observed screening times and hospital administrators' surveys, by nursery type and equipment type: January–February 2012
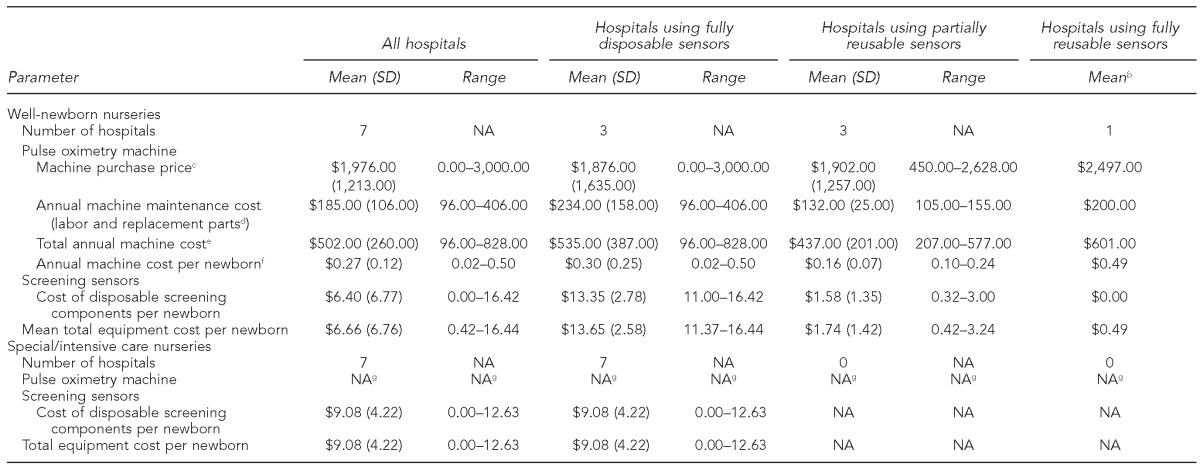
All estimates presented as 2011 values based on: Bureau of Labor Statistics (US). Producer price index industry data: hospitals. PCU622. 2011 [cited 2012 May 18]. Available from: URL: http://www.bls.gov/data/#prices
bSDs and ranges not included because only one hospital used fully reusable sensors.
cMachines purchased in earlier years were inflated to 2011 prices. Includes one hospital that received free pulse oximetry machines for the well-newborn nursery with an accompanying contract with the same vendor to purchase disposable screening sensors.
dThe cost of replacement parts includes cords and reusable sensors.
eAssumes a seven-year useful life for pulse oximetry machines in well-newborn nurseries, which was the mean reported among the hospitals in the sample (range: 5–10 years). The amortized cost of the equipment was estimated using a discount rate of 3% and the method recommended in: Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005. The amortized equipment cost was summed with annual machine maintenance costs reported in the table to produce the estimated total annual machine cost.
fThe annual machine cost per newborn is the total annual machine cost divided by 93.3% of the 2010 birth census count for each hospital from the New Jersey Department of Health (birth counts are not reported to protect the anonymity of hospitals that participated in the evaluation), based on a national estimate that 6.7% of newborns are admitted to special/intensive care nurseries (see: Osterman MJ, Martin JA, Mathews TJ, Hamilton BE. Expanded data from the new birth certificate, 2008. Natl Vital Stat Rep 2011 Jul 27;59[7]:1-28). This approach assumes that hospitals needed to purchase one additional pulse oximetry machine for CCHD screening in the well-newborn nursery.
gAll hospitals observed in the evaluation used existing vital signs equipment in special/intensive care nurseries to take pulse oximetry readings for CCHD screening.
CCHD = critical congenital heart disease
SD = standard deviation
NA = not applicable
Special/intensive care nurseries.
All hospitals in the sample used fully disposable sensors for CCHD screening in special/intensive care nurseries (Table 2). Hospitals' incremental cost for disposable sensors for each newborn ranged from zero dollars to $12.63. In the hospital with zero cost for disposable screening equipment, staff transferred a disposable sensor that was already being used for newborns' continuous vital signs monitoring to the newborn's other extremity for the second reading required for the CCHD screening. At the hospital with the highest cost for disposable screening equipment, staff used an additional disposable sensor on the newborn's arm for the second reading.
Equipment costs
At all hospitals, well-newborn nurseries used pulse oximetry machines prior to the CCHD screening requirement. Three of the seven hospitals purchased an additional machine for well-newborn nurseries to accommodate routine CCHD screening. To be conservative, our cost estimates assumed that hospitals needed to purchase one additional pulse oximetry machine for routine CCHD screening in well-newborn nurseries (an average annual amortized machine cost of $0.17 [SD=$0.12] per newborn) (data not shown). We assumed no equipment purchases in special/intensive care nurseries because existing equipment was used to perform the CCHD screening at all hospitals we visited.
As shown in Table 2, fixed equipment costs and the cost of disposable screening components are reported separately for well-newborn and special/intensive care nurseries. Hospitals' estimated total equipment cost per well-newborn screened was $6.66 (SD=$6.76, range: $0.42–$16.44). The estimated machine cost per well-newborn was low for all hospitals, ranging from $0.02 to $0.50; therefore, the variation in total equipment costs was mainly due to disposable vs. reusable screening sensors. Hospitals that used fully disposable sensors (n=3) had the highest estimated total equipment cost per well-newborn (mean = $13.35, SD=$2.78, range: $11.00–$16.42), while the hospital that used fully reusable sensors had the lowest estimated cost per well-newborn screened (mean = $0.49; measures of dispersion not applicable). Hospitals' estimated total equipment cost per newborn screened in special/intensive care nurseries was $9.08 (SD=$4.22), which was the mean cost of disposable screening components.
Total costs
The total estimated cost for CCHD screening in well-newborn nurseries in New Jersey was $14.09 (consisting of $7.43 in labor and $6.66 in equipment and supplies). The total estimated cost for screening in special/intensive care nurseries in New Jersey was $14.70 ($5.62 in labor and $9.08 in equipment and supplies). The weighted average cost to screen each newborn, regardless of nursery facility, was an estimated $14.19 ($7.36 in labor and $6.83 in equipment and supplies) in New Jersey and $13.50 ($6.68 in labor and $6.83 in equipment and supplies) in the U.S. (data not shown). We did not pursue further aggregate calculations based on hospital-specific measures due to the small number of observations in the sample.
DISCUSSION
The estimated cost in New Jersey of more than $14 per newborn for hospital-based CCHD screening using pulse oximetry is comparable with published reports of other newborn screening tests. It is less than the estimated cost of $20 per newborn for laboratory metabolic screening18 and less than the $36–$39 cost per newborn for hospital-based hearing screening.19 However, this evaluation's estimated cost per newborn for CCHD screening is higher than the estimated cost of $5 for laboratory testing for severe combined immunodeficiency screening (SCID).20 These cost estimates for metabolic screening, hearing, and SCID screening exclude follow-up costs, such as further diagnostic testing, as well as administrative overhead costs.
The screening cost estimate reported in this article was a critical input in a cost-effectiveness analysis.21 Based on other published information, such as screening sensitivity and specificity,22 that analysis estimated that screening in all U.S. hospitals could detect an additional 1,189 newborns with CCHD that would otherwise leave birth hospitals without a diagnosis. The net cost of screening was estimated to be $6.28 per newborn, which includes the cost of screening reported here and anticipated savings in hospital costs due to early detection that were reported elsewhere.23 The estimated net medical cost to detect one newborn with CCHD through screening was $20,862 because hospitals need to screen many newborns to find one newborn with CCHD. Screening was projected to avert up to 20 infant deaths per year, at a favorable cost-effectiveness estimate of $42,385 per life-year gained.
Our estimate of CCHD screening equipment costs is concordant with a previous estimate that accounted for the use of disposable pulse oximetry sensors.6 The labor time required per screen reported in this evaluation is greater than was reported in the recent U.S. literature;3,5,6,13–15 however, as noted previously, prior estimates were not based on reported systematic studies conducted by objective observers. An evaluation in Georgia hospitals applied the methods developed for the present evaluation and reported a mean screening time estimate of 10 minutes per newborn.24
Previous screening time estimates may have considered only the time it takes to obtain a pulse oximetry reading once the sensor has been attached to a newborn. In daily practice in the hospitals visited, several other activities related to CCHD screening occupied nurses' time. Activities directly related to the screening included transit time to bring a baby to the nursery if newborns were with mothers in postpartum rooms (and if CCHD screening was not bundled with existing newborn metabolic and/or hearing screening), gathering required equipment, and calming the infant to get a consistent pulse oximetry reading. Additional activities included transferring the sensor from the hand to the foot (if the nursery used the same sensor for two readings), disposing of or cleaning equipment, swaddling the infant, and recording the screening results. It would be conceptually incorrect to ignore the time it takes to complete all of these activities in estimates of the marginal labor time required to screen newborns for CCHD. There were many factors that influenced the screening time for each observed screening and the observers did not believe any one factor had a greater influence on the screening timings. Moreover, this evaluation comprised a small number of observed screenings; thus, results were not stratified by any of these factors. During the observations, no newborns required a repeat screen, which would have increased the observed screening time. However, low blood oxygen saturation in newborns detected through routine pulse oximetry screening is rare,25 and it is anticipated that required repeat screens would have a minimal impact on the mean screening times reported in this article.
Hospitals' estimated equipment costs per newborn screened varied substantially based on the type of pulse oximetry technology that hospitals used. This evaluation did not investigate clinical motivations for using exclusively disposable sensors for newborn CCHD screening. This evaluation also did not investigate the efficiency of specific pulse oximetry technologies. It is possible, for example, that disposable and reusable sensors could require more or less time to obtain an accurate pulse oximetry reading. This concept would be best tested in a clinical comparative-effectiveness trial of pulse oximetry sensors and machines.
Further cost studies can shed light on factors not included in this analysis, including the costs of nurse training and educational materials. Screening costs in other states may differ due to variations in CCHD screening mandates and practices. Our evaluation was conducted soon after CCHD screening was implemented in New Jersey; hospital costs may decrease over time as hospitals gain experience and potentially modify screening practices. Other factors that might affect screening time and cost include screening documentation procedures (such as paper vs. electronic medical records), the impact of transit time to the nursery for screening newborns that are roomed in with the mother, and the cost of hospital managers' time to administer the screening program.
One strength of this evaluation was that the criteria for screening time was applied consistently to all observed screenings by the same two researchers. The estimates of screening time presented in this article, therefore, may be more internally valid than estimates derived from clinician surveys. Another strength of this evaluation was its random sampling frame, which was made possible through New Jersey's mandate for CCHD screening. The cost estimates reported in this article assume that all inputs used in screening, including staff time, have an opportunity cost equal to their market price or cost. In particular, it is assumed that staff time spent in CCHD screening would otherwise have been spent in productive activities. That assumption may not be correct if screening does not displace productive work, in which case the true cost of labor time could be minimal. In the New Jersey hospital sample, nurses were able to fit the screening into their work schedules without adding paid hours of work. Therefore, the net cost of screening from the hospitals' own (accounting) perspective could be substantially lower than is reported in this article.
CONCLUSION
This evaluation addressed an issue that has been the subject of conjecture by providing the first direct observation in the U.S. of time and total cost for CCHD screening for a random, statewide sample of birth hospitals in New Jersey. Results suggest that hospitals' costs for pulse oximetry screening for CCHD are comparable with estimated costs for some existing newborn screening conditions. Decisions on pulse oximetry screening sensors can have a substantial effect on hospitals' costs; estimated equipment costs varied from $0.49 per newborn with reusable sensors to $13.65 per newborn with disposable sensors. These results might be used to inform policymaking in states considering screening mandates and might also be of interest to hospital administrators considering CCHD screening even in the absence of a mandate.
Footnotes
The authors acknowledge the hospital and nursing administrators and staff of New Jersey birthing hospitals for their participation in this project.
This evaluation was reviewed by the Institutional Review Board (IRB) Human Subjects contacts at the Centers for Disease Control and Prevention's (CDC's) National Center on Birth Defects and Developmental Disabilities and the New Jersey Department of Health (NJDOH). The study was determined by both agencies to qualify as public health practice and, therefore, was exempt from IRB review.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of NJDOH or CDC.
REFERENCES
- 1.Mahle WT, Martin GR, Beekman RH, 3rd, Morrow WR Section on Cardiology Cardiac Surgery Executive Committee. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129:190–2. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 2. N.J. Pub. L. No. 2011 Ch. 74, C.26:2-111.3, C26:2-111.4 (2011)
- 3.Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120:447–58. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- 4.Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128:e1259–67. doi: 10.1542/peds.2011-1317. [DOI] [PubMed] [Google Scholar]
- 5.Liske MR, Greeley CS, Law DJ, Reich JD, Morrow WR, Baldwin HS, et al. Report of the Tennessee Task Force on Screening Newborn Infants for Critical Congenital Heart Disease. Pediatrics. 2006;118:e1250–6. doi: 10.1542/peds.2005-3061. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman JI. It is time for routine neonatal screening by pulse oximetry. Neonatology. 2011;99:1–9. doi: 10.1159/000311216. [DOI] [PubMed] [Google Scholar]
- 7.Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C. Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2005;9:1–152. iii–iv. doi: 10.3310/hta9440. [DOI] [PubMed] [Google Scholar]
- 8.Griebsch I, Knowles RL, Brown J, Bull C, Wren C, Dezateux CA. Comparing the clinical and economic effects of clinical examination, pulse oximetry, and echocardiography in newborn screening for congenital heart defects: a probabilistic cost-effectiveness model and value of information analysis. Int J Technol Assess Health Care. 2007;23:192–204. doi: 10.1017/S0266462307070304. [DOI] [PubMed] [Google Scholar]
- 9.Roberts TE, Barton PM, Auguste PE, Middleton LJ, Furmston AT, Ewer AK. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a cost-effectiveness analysis. Arch Dis Child. 2012;97:221–6. doi: 10.1136/archdischild-2011-300564. [DOI] [PubMed] [Google Scholar]
- 10.Ewer AK, Furmston AT, Middleton LJ, Deeks JJ, Daniels JP, Pattison HM, et al. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess. 2012;16:v–xiii. 1–184. doi: 10.3310/hta16020. [DOI] [PubMed] [Google Scholar]
- 11.Organisation for Economic Co-operation and Development. OECD statistics [cited 2013 Sep 23] Available from: URL: http://stats.oecd.org.
- 12.Bureau of Labor Statistics (US) Producer price index industry data: hospitals. PCU622 2011 [cited 2012 May 18]. Available from: URL: http://www.bls.gov/data/#prices.
- 13.Walsh W. Evaluation of pulse oximetry screening in middle Tennessee: cases for consideration before universal screening. J Perinatol. 2011;31:125–9. doi: 10.1038/jp.2010.70. [DOI] [PubMed] [Google Scholar]
- 14.Koppel RI, Druschel CM, Carter T, Goldberg BE, Mehta PN, Talwar R, et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111:451–5. doi: 10.1542/peds.111.3.451. [DOI] [PubMed] [Google Scholar]
- 15.Bradshaw EA, Martin GR. Screening for critical congenital heart disease: advancing detection in the newborn. Curr Opin Pediatr. 2012;24:603–8. doi: 10.1097/MOP.0b013e328357a843. [DOI] [PubMed] [Google Scholar]
- 16.Osterman MJ, Martin JA, Mathews TJ, Hamilton BE. Expanded data from the new birth certificate, 2008. Natl Vital Stat Rep. 2011 Jul 27;59(7):1–28. [PubMed] [Google Scholar]
- 17.Bureau of Labor Statistics (US) Occupational employment and wages, May 2011: 29-1111 registered nurses. 2011. [cited 2013 Sep 19]. Available from: URL: http://www.bls.gov/oes/2011/may/oes 291111.htm.
- 18.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(5 Pt 2):S287–95. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 19.Vohr BR, Oh W, Stewart EJ, Bentkover JD, Gabbard S, Lemons J, et al. Comparison of costs and referral rates of 3 universal newborn hearing screening protocols. J Pediatr. 2001;139:238–44. doi: 10.1067/mpd.2001.115971. [DOI] [PubMed] [Google Scholar]
- 20.Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID) Mol Genet Metab. 2011;104:383–9. doi: 10.1016/j.ymgme.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson C, Grosse SD, Oster ME, Olney RS, Cassell CH. Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatrics. 2013;132:595–603. doi: 10.1542/peds.2013-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379:2459–64. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 23.Peterson C, Dawson A, Grosse SD, Riehle-Colarusso T, Olney RS, Tanner JP, et al. Hospitalizations, costs, and mortality among infants with critical congenital heart disease: how important is timely detection? Birth Defects Res A Clin Mol Teratol. 2013;97:664–72. doi: 10.1002/bdra.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assessment of current practices and feasibility of routine screening for critical congenital heart defects—Georgia, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(15):288–91. [PMC free article] [PubMed] [Google Scholar]
- 25.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Accuracy of pulse oximetry in screening for congenital heart disease in asymptomatic newborns: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2007;92:F176–80. doi: 10.1136/adc.2006.107656. [DOI] [PMC free article] [PubMed] [Google Scholar]


