Abstract
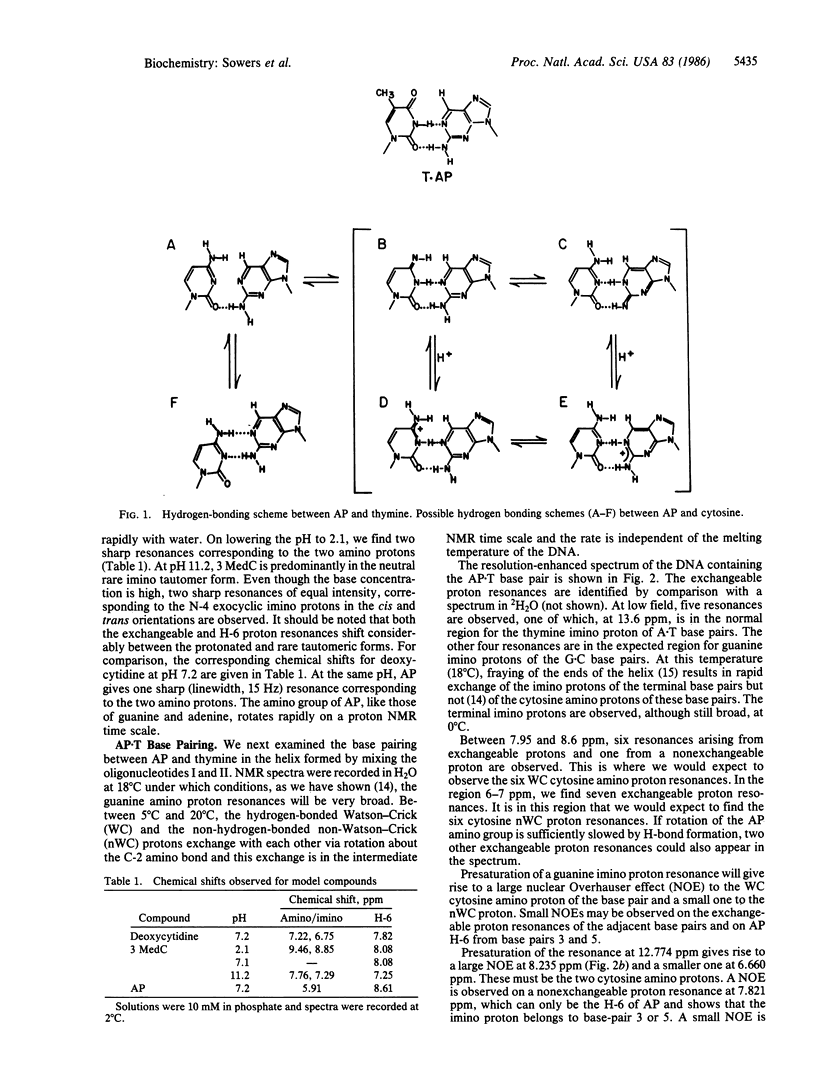
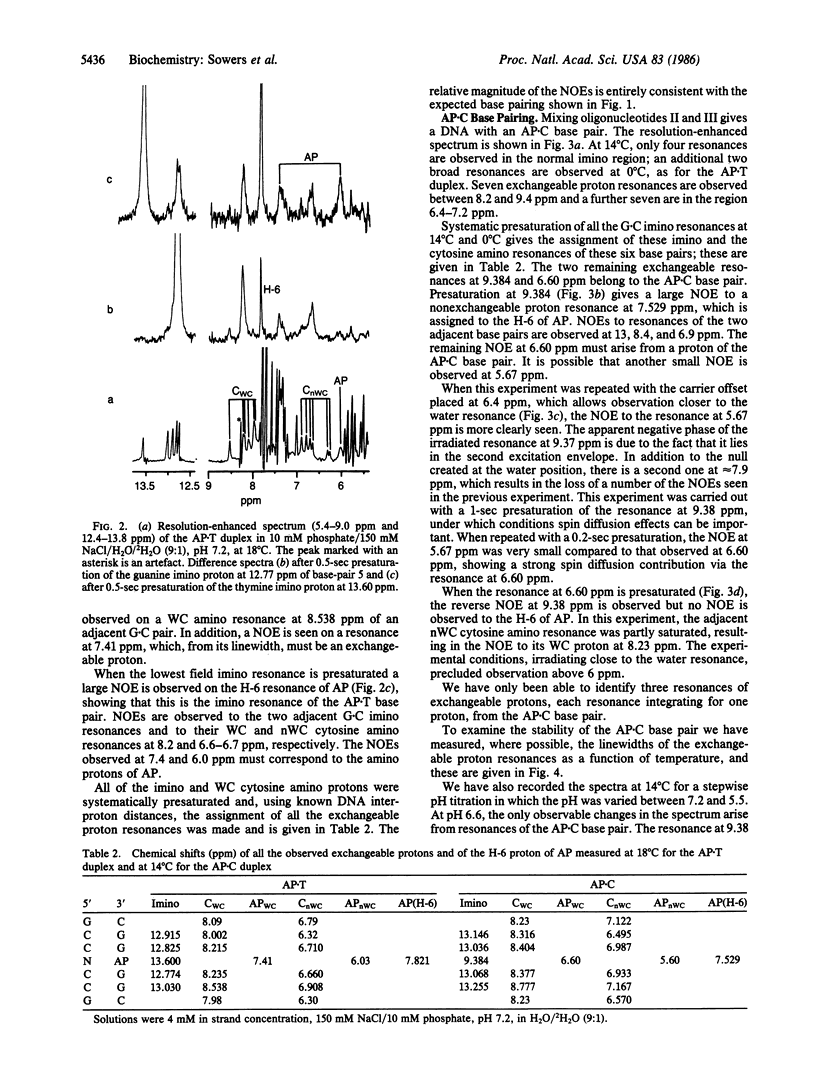
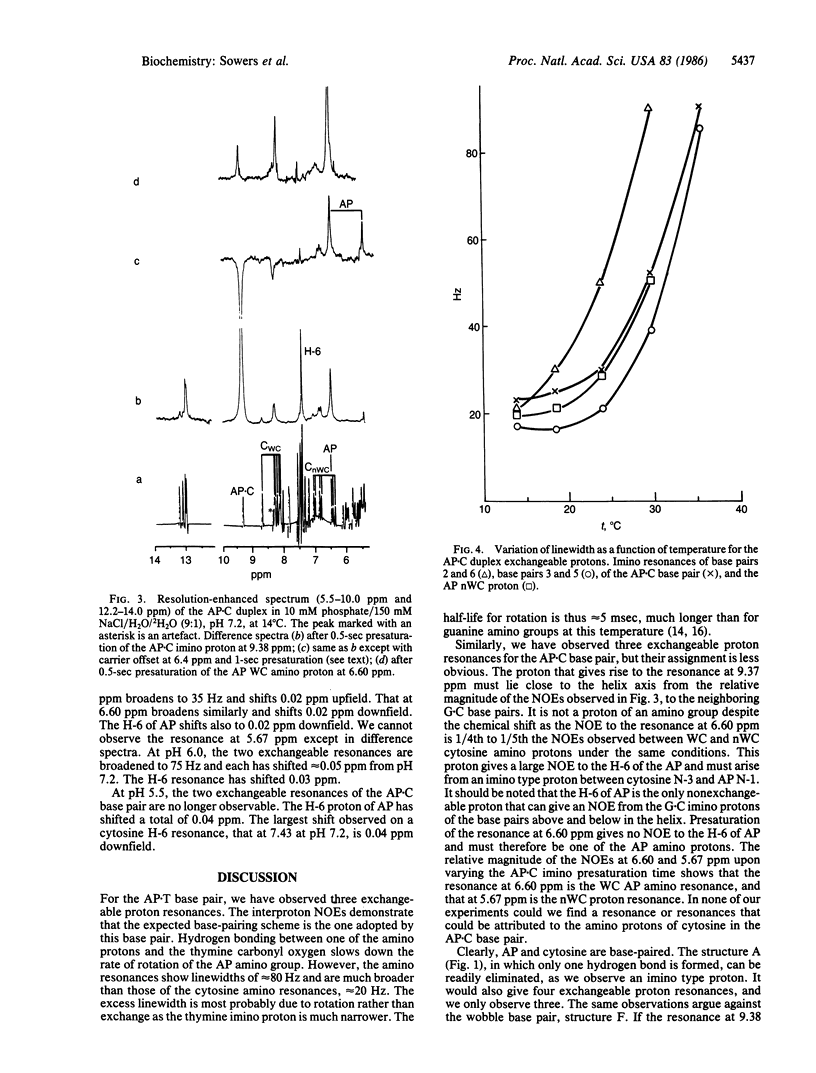
2-Aminopurine (AP), a potent mutagenic base analogue, most frequently pairs with thymine. In the AP X T base pair, both bases adopt normal tautomeric forms. The mechanism for the mutagenic activity arises from its observed pairing with cytosine, which has been ascribed to an enhanced tendency to adopt the rare imino tautomeric form. NMR studies in H2O on all the exchangeable protons in an oligonucleotide duplex containing an AP X T base pair show Watson-Crick hydrogen bonding. When the thymine is replaced by cytosine in the duplex, we observe an AP X C base pair. Both amino protons of AP are seen excluding the rare tautomeric form. Although several alternative structures are possible, it is shown that the second hydrogen bond is formed by protonation of the AP X C base pair and that this is the dominant species under physiological conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessman M. J., Muzyczka N., Goodman M. F., Schnaar R. L. Studies on the biochemical basis of spontaneous mutation. II. The incorporation of a base and its analogue into DNA by wild-type, mutator and antimutator DNA polymerases. J Mol Biol. 1974 Sep 15;88(2):409–421. doi: 10.1016/0022-2836(74)90491-4. [DOI] [PubMed] [Google Scholar]
- Brown T., Kennard O., Kneale G., Rabinovich D. High-resolution structure of a DNA helix containing mismatched base pairs. Nature. 1985 Jun 13;315(6020):604–606. doi: 10.1038/315604a0. [DOI] [PubMed] [Google Scholar]
- Courtois Y., Fromageot P., Guschlbauer W. Protonated polynucleotide structures. 3. An optical rotatory dispersion study of the protonation of DNA. Eur J Biochem. 1968 Dec 5;6(4):493–501. doi: 10.1111/j.1432-1033.1968.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Danilov V. I., Krugliak Iu A., Kuprievich V. A., Shramko O. V. O mekhanizme mutagennogo deistviia 2-aminopurina. Biofizika. 1967 Jul-Aug;12(4):726–729. [PubMed] [Google Scholar]
- Dattagupta N., Crothers D. M. Solution structural studies of the Ag(I)-DNA complex. Nucleic Acids Res. 1981 Jun 25;9(12):2971–2985. doi: 10.1093/nar/9.12.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn G. L., Butzow J. J., Clark P., Tarien E. Interaction of metal ions with polynucleotides and related compounds. X. Studies on the reaction of silver (I) with the nucleosides and polynucleotides, and the effect of silver(I) on the zinc(II) degradation of polynucleotides. Biopolymers. 1967 Mar;5(3):283–296. doi: 10.1002/bip.1967.360050306. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V., Téoule R., Guy A., Fritzsche H., Guschlbauer W. NMR studies on oligodeoxyribonucleotides containing the dam methylation site GATC. Comparison between d(GGATCC) and d(GGm6ATCC). Biochemistry. 1985 Aug 13;24(17):4540–4548. doi: 10.1021/bi00338a009. [DOI] [PubMed] [Google Scholar]
- Fazakerley G. V., van der Marel G. A., van Boom J. H., Guschlbauer W. Helix opening in deoxyribonucleic acid from a proton nuclear magnetic resonance study of imino and amino protons in d(CG)3. Nucleic Acids Res. 1984 Nov 12;12(21):8269–8279. doi: 10.1093/nar/12.21.8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. F., Ratliff R. L. Evidence of 2-aminopurine-cytosine base mispairs involving two hydrogen bonds. J Biol Chem. 1983 Nov 10;258(21):12842–12846. [PubMed] [Google Scholar]
- Hilbers C. W., Patel D. J. Proton nuclear magnetic resonance investigations of the nucleation and propagation reactions associated with the helix-coil transition of d-ApTpGpCpApT in H2O solution. Biochemistry. 1975 Jun 17;14(12):2656–2660. doi: 10.1021/bi00683a015. [DOI] [PubMed] [Google Scholar]
- McConnell B. Exchange mechanisms for hydrogen bonding protons of cytidylic and guanylic acids. Biochemistry. 1978 Jul 25;17(15):3168–3176. doi: 10.1021/bi00608a035. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Marky L. A., Rice J. A., Broka C., Dallas J., Itakura K., Breslauer K. J. Structure, dynamics, and energetics of deoxyguanosine . thymidine wobble base pair formation in the self-complementary d(CGTGAATTCGCG) duplex in solution. Biochemistry. 1982 Feb 2;21(3):437–444. doi: 10.1021/bi00532a003. [DOI] [PubMed] [Google Scholar]
- RUDNER R. Mutation as an error in base pairing. Biochem Biophys Res Commun. 1960 Sep;3:275–280. doi: 10.1016/0006-291x(60)90239-4. [DOI] [PubMed] [Google Scholar]
- Ronen A. 2-Aminopurine. Mutat Res. 1980 Jan;75(1):1–47. doi: 10.1016/0165-1110(80)90026-3. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953 May 30;171(4361):964–967. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. The structure of DNA. Cold Spring Harb Symp Quant Biol. 1953;18:123–131. doi: 10.1101/sqb.1953.018.01.020. [DOI] [PubMed] [Google Scholar]
- Watanabe S. M., Goodman M. F. On the molecular basis of transition mutations: frequencies of forming 2-aminopurine.cytosine and adenine.cytosine base mispairs in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2864–2868. doi: 10.1073/pnas.78.5.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]



