Abstract
We present a female patient in her late 30s, with baseline vegetative state following prior traumatic brain injury, who presented with prolonged right hemispheric status epilepticus. The neuroimaging revealed a striking right-sided pancortical oedema with left (crossed) cerebellar diaschisis and dilation of right hemispheric arteries. EEG was concordant and showed nearly continuous right hemispheric seizure discharges with suppressed background. Infective and vascular aetiologies were ruled out. The patient showed clinical and electrographic improvement following treatment with antiepileptic drugs. Unilateral cerebral oedema is a rare presentation of focal status epilepticus, and should be considered as a differential diagnosis in the appropriate clinical scenario.
Background
A host of neuroimaging changes related to status epilepticus have been described.1 These include peri-ictal and postictal changes. With the availability of MRI scans, the rare peri-ictal abnormalities are now increasingly recognised. These periictal imaging changes can be both local (related to seizure discharge zone) and remote (in distant structure that may trans-synaptically connect to the discharge zone). The local structural changes that have been reported are hippocampal and focal cortical oedema.1 However, unilateral panhemispheric changes with midline shift have never been described to our knowledge. Also, focal changes are often misdiagnosed to be space-occupying lesions, evolving ischaemia or cerebritis, and finding the right aetiology is often challenging. We present such a patient who presented with focal status epilepticus and describe the clinicoimaging and EEG findings.
Case presentation
A female patient in her late 30s was presented with a vegetative state at her baseline following a severe traumatic brain injury (TBI) due to a road traffic accident (RTA) 5 years prior to presentation. She was brought to the hospital by her mother with a 1-week history of progressive left-sided jerking that started as left face and eye twitching then progressed to involve the left upper and lower limbs. The jerking initially lasted for 1–2 min but eventually the duration prolonged and it lasted for over 15–30 min. The frequency also has increased gradually, initially it was occurring 1–3 times/day but in the last 2 days before presentation, the seizures were almost continuous. In addition, as per the mother, the patient's mental status had declined in the last 2–3 days prior to presentation as she was not opening her eyes, moving her extremities or vocalising as before. There was no history of associated fever, vomiting, cough or recent trauma.
Her medical history is significant for severe TBI 5 years ago resulting in a vegetative state. The type of TBI was probably a combination of diffuse axonal injury and cerebral contusion. This is based on the review of the available CT scan carried out 1 year prior to presentation (figure 1A), that showed thinning of the cortex (suggestive of cortical injury) and diffuse brain atrophy suggestive of bilateral damage to the white matter.
Figure 1.
(A) Plain axial head CT carried out 1 year prior to presentation showing generalised atrophy related to severe brain injury. (B) Plain axial head CT at presentation showing the right hemispheric oedema and midline shift.
The scans carried out at the time of the RTA were not available. Currently, the patient is bed-ridden with percutaneous endoscopic gastrostomy tube feeding and suprapubic urinary catheter. She would blink eyes, with only some occasional vocalisations. The patient also suffered from few episodes of seizures within the first week of the TBI and was treated with antiepileptic medications for about 1 year and then medications stopped after that. The patient was also diagnosed with hypothyroidism a few months following the accident and was put on thyroxine.
Family history is negative for febrile convulsions, epilepsy or any other neurological disorders. She is the eldest of nine siblings and used to work as a teacher before the accident. No history of smoking, alcohol or illicit drug use was reported. Her mother is her caregiver.
On presentation to the emergency room, the patient underwent a CT scan followed by treatment of the seizures with intravenous benzodiazepines and phenytoin that caused hypotension. On examination, the vitals were blood pressure 73/52 mm Hg, pulse rate 88 bpm, respiratory rate 18 breaths/min, oxygen saturation of 100% on room air and temperature 36.6.
The patient's eyes were closed, she was unresponsive to commands and her pupils were 3 mm in size with a sluggish reaction to light bilaterally. A funduscopic examination did not reveal any retinal bleeding or papilloedema. Patient moved her limbs to pain bilaterally. Also, she had facial griming and was vocalising to pain. Reflexes are brisk diffusely and the plantar was upgoing on the left side and equivocal on the right side.
The patient was started on vasopressors and admitted to the intensive care unit (ICU) for further management.
Investigations
Blood tests at admission revealed normal complete blood count, liver and renal functions, blood glucose and calcium levels. Electrolytes panel showed mild hyponatraemia of 134 mmol/L, hyperkalaemia of 6.1 mmol/L and normal bicarbonate level of 27 mmol/L.
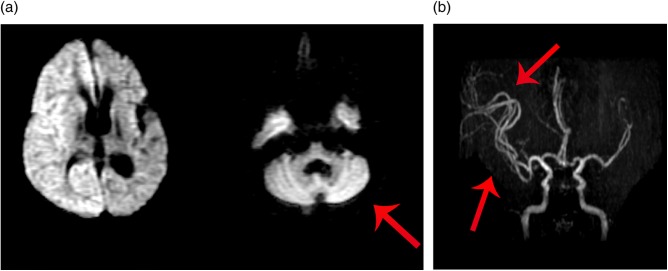
A plain CT of the brain at the time of admission revealed new effacement of the right-sided cortical sulci and signs of mass effect and some effacement of the right lateral ventricle and mild midline shift to the left side. The left hemisphere did not reveal any new changes (figure 1A, B). An MRI of the brain carried out 1 day later showed right pancortical and left (contralateral) cerebellar restriction on diffusion-weighted images (DWI) and hyperintense signals and oedema in corresponding areas on fluid attenuated inversion recovery (FLAIR) images. These findings are consistent with crossed cerebellar diaschisis (figure 2A). In addition, the MR angiogram (MRA) revealed increased dilation of arteries supplying the right hemisphere (figure 2B).
Figure 2.
(A) Diffusion-weighted image axial brain MRI showing right hemisphere and with contralateral cerebellar hyperintensity (crossed cerebellar diaschisis). (B) MR angiogram of the brain showing dilated right-sided arteries.
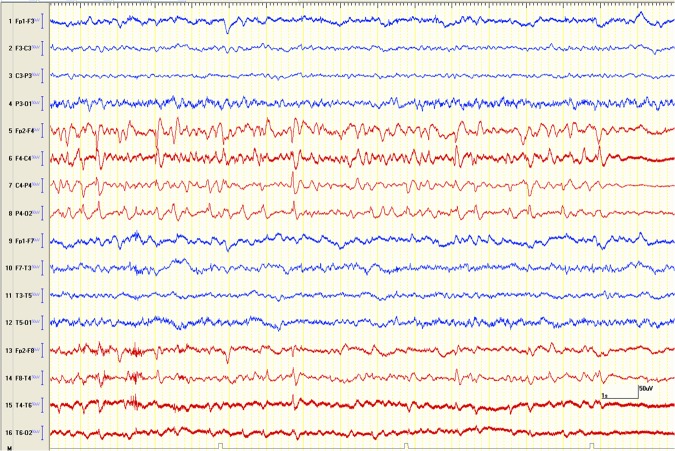
An EEG carried out on day 2 of admission revealed the presence of nearly continuous subclinical seizure discharges over the right hemisphere. This finding would be consistent with focal (non-convulsive) status epilepticus. In addition, a moderately severe degree of diffuse slow wave abnormalities were seen that would be suggestive of generalised or multifocal non-specific encephalopathy consistent with prior severe TBI (figure 3).
Figure 3.
Longitudinal bipolar EEG epoch, on presentation, showing the right hemispheric (the red coloured channels) seizure followed by background suppression.
A lumbar puncture on day 3 was completely normal with white blood cells: 0, red blood cells: 0, protein: 293 mg/L and glucose: 3.6 mmol/L.
PCRs for enteroviruses, herpes simplex virus and herpes zoster were negative. Cerebrospinal fluid (CSF) Gram stain and culture did not grow any organism. CSF bacterial antigen and cryptococcal antigen tests were negative as well.
Differential diagnosis
Differential diagnoses for unilateral brain oedema are relatively few. These may include:
Viral encephalitis: Our patient did not have any history suggestive of fever. Also, malignant encephalitis as caused by herpes simplex virus involves bilateral brain with predilection for frontotemporal and insular areas. The CSF findings including PCR for common viruses were negative.
Ischaemic stroke: Clinically, as the patient had baseline quadriparesis, stroke could not be ruled out. However, the mother did not notice more than usual weakness involving the left side. Also, the lesion seen on the scan is not restricted to a vascular distribution. Lastly, an MRA revealed patent and increased arterial circulation over the right hemisphere.
Central nervous system vasculitis can present in varied ways. However the common presentations are multifocal ischaemia and haemorrhages. In our patient, this was not the case; in addition, the CSF did not show any protein elevations and MRA (even though not the gold standard for diagnosis) did not show any evidence of vasculitis.
Cerebral anoxia can have clinical presentation of seizures and MRI changes similar to our patient. However, both sides of the brain would be expected to be involved. In addition, no history of prehospital cardiorespiratory arrest was present.
Possible paraneoplastic/autoimmune aetiologies were also considered, however less likely in the absence of relevant history and negative CSF findings.
Metabolic or toxic aetiologies are also less likely in the absence of history or lab findings. Also strictly unilateral brain involvement would be extremely rare.
Treatment
In the emergency room, prior to being seen by the neurology team, the patient received intravenous lorazepam, diazepam and loading dose of phenytoin. Following these, her clinical seizures stopped, however she developed hypotension for which she was started on dopamine infusion and transferred to the ICU for further care. In addition, the patient was given intravenous antibiotics for possible meningoencephalitis.
In ICU, phenytoin was replaced by levetiracetam initially at a dose of 500 mg twice daily, and later increased to 1000 mg twice daily as the bedside EEG showed presence of nearly continuous right hemispheric subclinical electrographic seizure discharges (figure 3). Once the CSF results were found to be normal, the antibiotics were stopped.
Outcome and follow-up
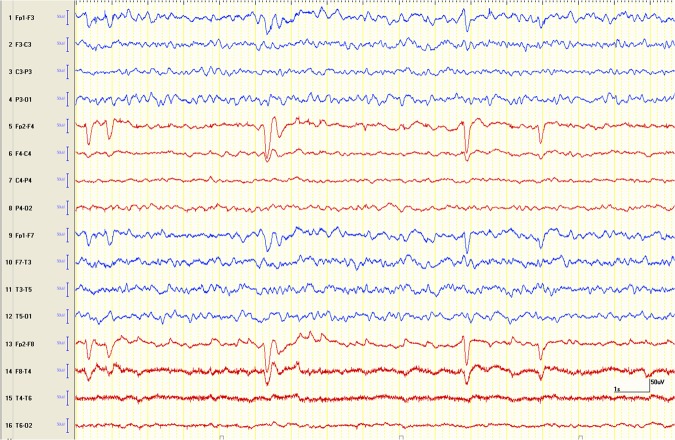
The patient improved clinically and on day 6 of admission was almost at her baseline. Repeat EEG carried out on day 7 showed an absence of right-sided seizure discharge and improvement of background rhythm. However, the cerebral activity on the right side continued to be suppressed (figure 4). A repeat MRI carried out on day 8 showed a trend towards improvement of cerebral oedema and midline shift (figure 5A, B). The patient was discharged home on day 9 to be followed as an outpatient with a plan to repeat MRI to see if the patient would develop atrophy in the affected hemisphere.
Figure 4.
Post-treatment longitudinal bipolar EEG epoch showing resolution of the right hemispheric (red coloured channels) seizure discharge with some recovery of background activity.
Figure 5.
(A) Preseizure treatment T2-weighted coronal brain MRI showing the right hemispheric oedema and midline shift. (B) Follow-up brain MRI on day 8 post antiepileptic treatment showing marked decrease in brain oedema and resolution of midline shift.
Discussion
A wide spectrum of peri-ictal and postictal neuroimaging findings have been described in the literature.1–4 These findings can be classified as either local or remote, with respect to the site of seizure onset zone.1 Generally the local abnormalities are lobar and not panhemisphereic,1 as in our patient. In addition, we also found involvement of contralateral cerebellum, a very rare entity of remote findings called crossed cerebellar diaschisis phenomena, which refers to reduced metabolism and blood flow in the cerebellar hemisphere contralateral to a cerebral pathology (figure 2A), this remote finding is well observed in ischaemia5 6 but it is a quite rare finding in status epilepticus.1 5
The aetiology and pathophysiology of these neuroimaging changes remain unclear.1 7 However, few small studies carried out using multisequence MRI and MR spectroscopy showed intermixed areas of increased/decreased diffusivity associated with a lactate peak and decreased N-acetylaspartate, which suggest a cytotoxic oedema, this will be manifested by increased T2 signal intensity and restricted diffusion (bright on
DWI, dark on ADC maps).7 A vasogenic aetiology resulting from hypermetabolism associated with seizure activity related to the breakdown of the blood–brain barrier and ictal hyperperfusion has been described as well.7 8 In our patient the MRA findings of increased arterial circulation over the right hemisphere in response to the metabolic demands on that side in comparison to the left side (figure 2B) really support this theory.
The clinical and radiological findings in our case have a striking resemblance to those seen in hemiconvulsion-hemiplegia-epilepsy syndrome in the paediatric population,9 which is a rare outcome of prolonged focal febrile convulsions with hemiplegia and subsequent epilepsy later9; brain imaging of those patients showed initial unilateral oedema followed by cerebral hemiatrophy at a later stage.10 The aetiology is suggested to be mixture of inflammatory cytokines activation induced by the focal status epilepticus or presumed primary viral infection that leads to initial cytotoxic brain oedema and eventually neuronal cell necrosis and atrophy.10 Other studies suggested genetic predisposition in those children linked to CACNA1A gene mutation.11
What forms a real challenge is that status epilepticus-induced brain changes can sometimes mimic tumour or ischaemic lesions,1–3 which makes the diagnosis difficult and sometimes mandates careful and extensive workup for these patients. We faced that with our patient to a great extent; especially that she has a poor baseline mental and functional status. This made the assessment of new neurological deficit not as easy as it should be, but giving the unique panhemispheric changes in the MRI, which is not following a specific vascular distribution and the patent arterial circulation via MRA makes ischaemic aetiology less likely.
Unihemispheric acute infective encephalitis still remained ‘to be considered’ differential in our patient's case,12 so further investigations including a CSF examination on our patient were needed, but the normal results of CSF analysis and the negative PCR for common viral pathogens made an infectious process very less likely. Also, supporting that with EEG findings of continuous right hemispheric seizure discharges and clinical, electrical and radiological improvement following treatment with antiepileptic drugs alone made status epilepticus more likely to be the cause of the pan-unihemispheric oedema.
Chronic autoimmune encephalitis such as Rasmussen's encephalitis was also part of our differential diagnosis. It is a rare, chronic inflammatory neurological disease that usually presents during childhood.13 It is characterised by unihemispheric cortical inflammation and intractable focal seizures and epilepsia partialis continua. This over months and years leads to hemiparesis and cognitive deficits, and may progress to involve the other hemisphere.13 The MRI reveals cortical hyperintensity and gradual atrophy of the affected hemisphere.14 Our patient did not have any neurological problems prior to the RTA. Also neuroimaging on our patient revealed an acute unilateral oedema of the right cerebral hemisphere that started to resolve with stopping of clinical and electrographic seizures following optimisation of antiepileptic drugs (figure 5A,B). We had kept an extra CSF sample in the laboratory for additional testing of autoantibodies, and probably would have proceeded for brain biopsy in case patient did not improve.
Progressive mitochondrial disorders such as MELAS (myopathy, encephalopathy, lactic acidosis and stroke-like episodes) may present with episodic seizures. However, the hallmark is the stroke-like episodes with predominant involvement of posterior head regions. Also, such patients would present early in life often with a history of headaches, visual difficulties, developmental delay and learning disabilities.15 Our patient was completely normal prior to the RTA.
The long-term outcomes of status epilepticus-induced radiological changes remains uncertain,3 7 but theoretically status epilepticus-induced structural changes may be completely reversible if the seizures are treated promptly and optimally. A small prospective observational study followed those patients with similar radiological findings as our patient presenting good clinical outcomes, and complete resolution of neuroimaging findings with optimal treatment.16 17 This in general mandates early recognition of these changes and early treatment with antiepileptic drugs for better outcomes.2 3 7
Our case with the unique and rarity of its neuroradiological findings serves as reminder to consider focal status epilepticus as one of the differential diagnosis of unihemispheric brain oedema.
Learning points.
Acute peri-ictal imaging changes can occur with repetitive seizures or status epilepticus.
Seizures as the aetiology for these changes should be considered in the right clinical setting.
Early and effective treatment of seizure may reverse imaging finding and improve outcomes.
Footnotes
Contributors: This case was managed by SS and SKPC from the neurology department in Mafraq Hospital, SS gave the idea of publishing the case because of its rarity. NAA performed the literature research and wrote the article under the guidance of senior neurologists (SS and SKPC).
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cole AJ. Status epilepticus and periictal imaging. Epilepsia 2004;45(Suppl 4):72–7 [DOI] [PubMed] [Google Scholar]
- 2.Hicdonmez T, Utku U, Turgut N, et al. Reversible postictal MRI change mimicking structural lesion. Clin Neurol Neurosurg 2003;105:288–90 [DOI] [PubMed] [Google Scholar]
- 3.Canas N, Breia P, Soares P, et al. The electroclinical-imagiological spectrum and long-term outcome of transient periictal MRI abnormalities. Epilepsy Res 2010;91:240–52 [DOI] [PubMed] [Google Scholar]
- 4.Silverstein AM, Alexander JA. Acute postictal cerebral imaging. AJNR Am J Neuroradiol 1998;19:1485–8 [PMC free article] [PubMed] [Google Scholar]
- 5.Komaba Y, Mishina M, Utsumi K, et al. Crossed cerebellar diaschisis in patients with cortical infarction: logistic regression analysis to control for confounding effects. Stroke 2004;35:472–6 [DOI] [PubMed] [Google Scholar]
- 6.Di Piero V, Chollet F, Dolan RJ, et al. The functional nature of cerebellar diaschisis. Stroke 1990;21:1365–9 [DOI] [PubMed] [Google Scholar]
- 7.Canas N, Soares P, Calado S, et al. Pathophysiology and long-term outcome of reversible tumor-like lesions induced by presenting status epilepticus. J Neuroimaging 2010;20:169–74 [DOI] [PubMed] [Google Scholar]
- 8.Hong KS, Cho YJ, Lee SK, et al. Diffusion changes suggesting predominant vasogenic oedema during partial status epilepticus. Seizure 2004;13:317–21 [DOI] [PubMed] [Google Scholar]
- 9.Auvin S, Bellavoine V, Merdariu D, et al. Hemiconvulsion-hemiplegia-epilepsy syndrome: current understandings. Eur J Paediatr Neurol 2012;16:413–21 [DOI] [PubMed] [Google Scholar]
- 10.Tenney JR, Schapiro MB. Child neurology: hemiconvulsion-hemiplegia-epilepsy syndrome. Neurology 2012;79:e1–4 [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki S, Ikeno K, Abe T, et al. Hemiconvulsion-hemiplegia-epilepsy syndrome associated with CACNA1A S218L mutation. Pediatr Neurol 2011;45:193–6 [DOI] [PubMed] [Google Scholar]
- 12.Heo SH, Lee MS, Ahn TB, et al. A case of unilateral hemispheric encephalitis. Neurol Sci 2007;28:185–7 [DOI] [PubMed] [Google Scholar]
- 13.Bien CG, Widman G, Urbach H, et al. The natural history of Rasmussen's encephalitis. Brain 2002;125:1751–9 [DOI] [PubMed] [Google Scholar]
- 14.Chiapparini L, Granata T, Farina L, et al. Diagnostic imaging in 13 cases of Rasmussen's encephalitis: can early MRI suggest the diagnosis? Neuroradiology 2003;45:171–83 [DOI] [PubMed] [Google Scholar]
- 15.Hirano M, Pavlakis SG. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): current concepts. J Child Neurol 1994;9:4–13 [DOI] [PubMed] [Google Scholar]
- 16.Inamasu J, Nakamura Y, Yamamoto S, et al. Prolonged unilateral vasodilatation and brain edema in fulminant hepatic failure, associated with symptomatic seizure. Clin Neurol Neurosurg 2002;104:157–60 [DOI] [PubMed] [Google Scholar]
- 17.Carroll C, Krolikowski K, Mukonoweshuro W, et al. Unilateral cerebral hemisphere oedema as a peri-ictal phenomenon. J Neurol 2010;257:2094–6 [DOI] [PubMed] [Google Scholar]