Abstract
Perfluoroallyl chloride (PFAC), a fluorine-containing compound, has very severe toxicity, but this toxicity is not well characterised. We report a fatal case of acute chemical lung injury caused by the inhalation of PFAC. A 39-year-old man, working at a chemical factory, inhaled PFAC gas and died 16 days later of acute lung injury with severe pneumothorax. We present his clinical course together with thoracic CT findings, autopsy and analysis of PFAC in blood and urine samples with gas chromatograph–mass spectrometry. Previously, a fatal case of PFAC was reported in 1981 but PFAC was not identified in any of the patient's samples. In our patient, we identified PFAC in both blood and urine samples. Our toxicological analysis may be used as a reference to detect PFAC toxicity in the future. Our study should be helpful for diagnosing lung injury induced by a highly toxic gas, such as PFAC.
Background
Perfluoroallyl chloride (PFAC), listed in Chemical Abstracts Service (CAS) registry as No. 79–47–0, is an intermediate product of resin synthesis (figure 1).1 PFAC is used in the production of copolymers and elastomers.2 3 It is a precursor to (chlorodifluoromethyl) trifluorooxirane.4 PFAC has a similar chemical structure to allyl chloride. PFAC toxicity is very high because it is a fluorine-containing compound. Though many fatal cases due to other fluorine-containing compounds have been reported, there has been only one previous report on the toxicity of PFAC. Previously, a factory worker reportedly died of pneumonia but the likely cause based on his work environment was due to inhalation of PFAC.5 We report here a confirmed case of a patient who developed acute chemical lung injury due to PFAC inhalation.
Figure 1.
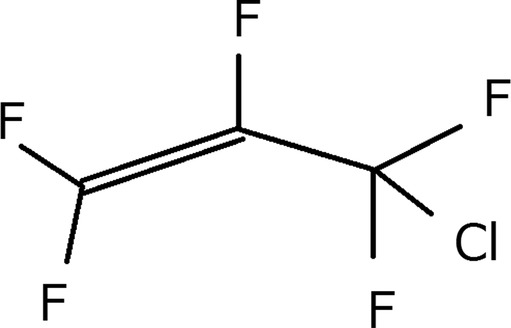
Perfluoroallyl chloride (PFAC structure). PFAC has all the hydrogens in allyl chloride replaced by fluorine.
Case presentation
A 39-year-old man, who worked at a chemical plant that synthesised resin for fuel cell membranes, was exposed to PFAC. He wore a gas mask and protective gloves for acids, but did not change his work clothes. He noticed the smell of a volatile solvent when he took off his mask. After 2 h, he had a slight fever. His symptoms progressed over the ensuing 24 h with shortness of breath, and he was subsequently admitted to our hospital.
Initial examination revealed paleness, diaphoresis, slight dyspnoea and a dry cough. Remarkable vital signs were as follows: respiratory rate 20/min; body temperature 38° and oxygen saturation 94% in room air. Respiratory sounds included crepitation on auscultation. Laboratory data showed elevated levels of white blood cells (17.9×103/μL) and C reactive protein (6.94 mg/dL). The other laboratory data were unremarkable.
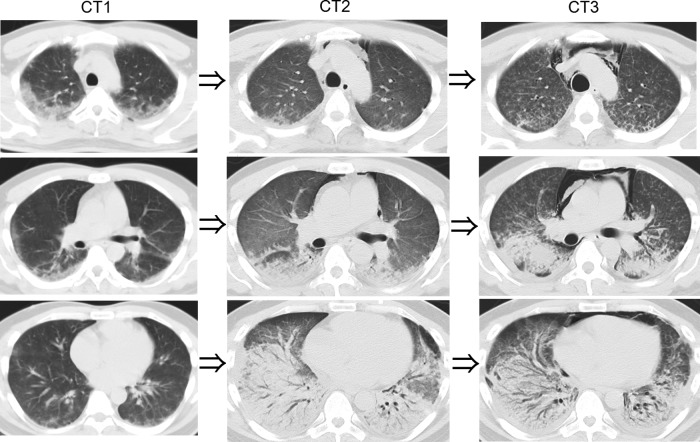
A chest X-ray did not show remarkable findings but a thoracic CT revealed areas of increased attenuation adjacent to pleura in the upper and middle fields of both lungs. In the lower fields, no opacities appeared (CT1, figure 2). Therapy for pneumonia was initiated with ceftriaxone. However, his respiratory condition deteriorated progressively and oxygen administration was needed. At that time, we considered him as having a chemical lung injury based on his work environment, as the timing of symptom onset and his clinical course were too rapid for bacterial pneumonia in such a young healthy person. After we collected his blood and urine samples for chemical analysis, we administered 1000 mg methylprednisolone for 3 days and his dyspnoea improved. A thoracic CT showed that the opacities in the upper field decreased but dense areas in the lower field, which were dominant on the dorsal side, appeared. Slight mediastinal emphysema also appeared (CT2, figure 2). Prednisolone at 80 mg/day (1.0 mg/kg) was started. On day 10, he increasingly suffered from laboured breathing. CT showed centrilobular nodules in the upper and middle lobes and the volume of the lower lobe decreased due to traction bronchiectasis. Furthermore, left and right pleural effusions appeared (CT3, figure 2). After confirming his normal circulatory system, we restarted steroid pulse therapy which was 500 mg methylprednisolone per 6 h for 3 days. However, no response was observed. He suffered from shortness of breath, and mechanical ventilation was started. Increasing levels in the concentration of inspired oxygen and positive end-expiratory pressure were required to sustain adequate oxygenation. On day 13, right-sided pneumothorax became obvious and mediastinal emphysema was widely spread. A chest tube was inserted but the air leakage continued spontaneously. On day 16, the patient died.
Figure 2.
CT on admission showed attenuation adjacent to the pleura in the upper and middle fields of both lungs (CT1). After steroid pulse therapy, the CT showed that the opacities decreased in the upper field but a denser area appeared in the lower field. Slight mediastinal emphysema also appeared (CT2). CT when the lung had lost a lot of function showed centrilobular nodules in the upper and middle lobes, and the volume of the lower lobe decreased due to traction bronchiectasis. Furthermore, left and right pleural effusions appeared (CT3).
Postmortem radiography was performed using CT. CT showed severe pneumothorax, mediastinal emphysema and pneumoderma. Both lungs were collapsed and lung fields could not be evaluated (figure 3).
Figure 3.
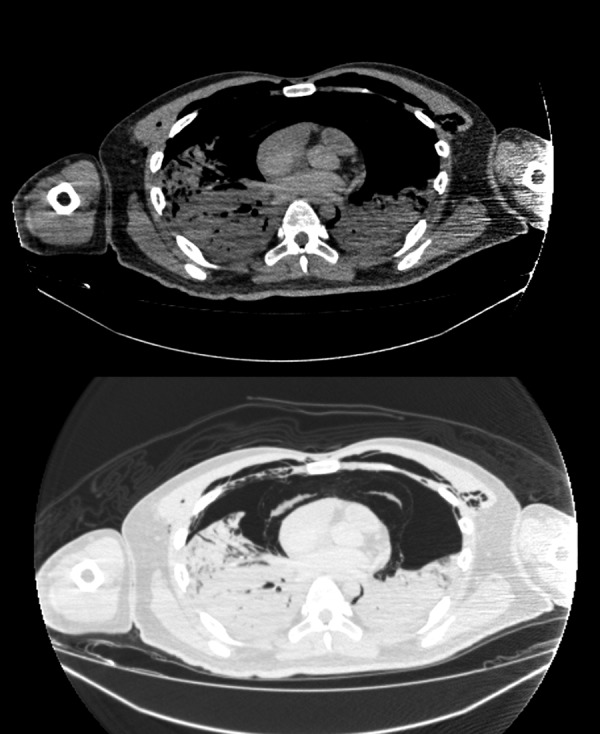
(Postmortem radiography) CT showed severe pneumothorax, mediastinal emphysema and pneumoderma. Both lungs were collapsed and lung fields could not be evaluated.
Investigations
Postmortem examination
We performed a forensic autopsy at the Department of Legal Medicine, Osaka University Graduate School of Medicine since the deceased had been suspected of being involved in an industrial accident. The autopsy revealed oedematous and haemorrhagic lungs (figure 4). The weights of the right and left lungs were 882 and 1272 g, respectively. This appearance is consistent with chemical lung injury features, but we could not see injury to the trachea.
Figure 4.
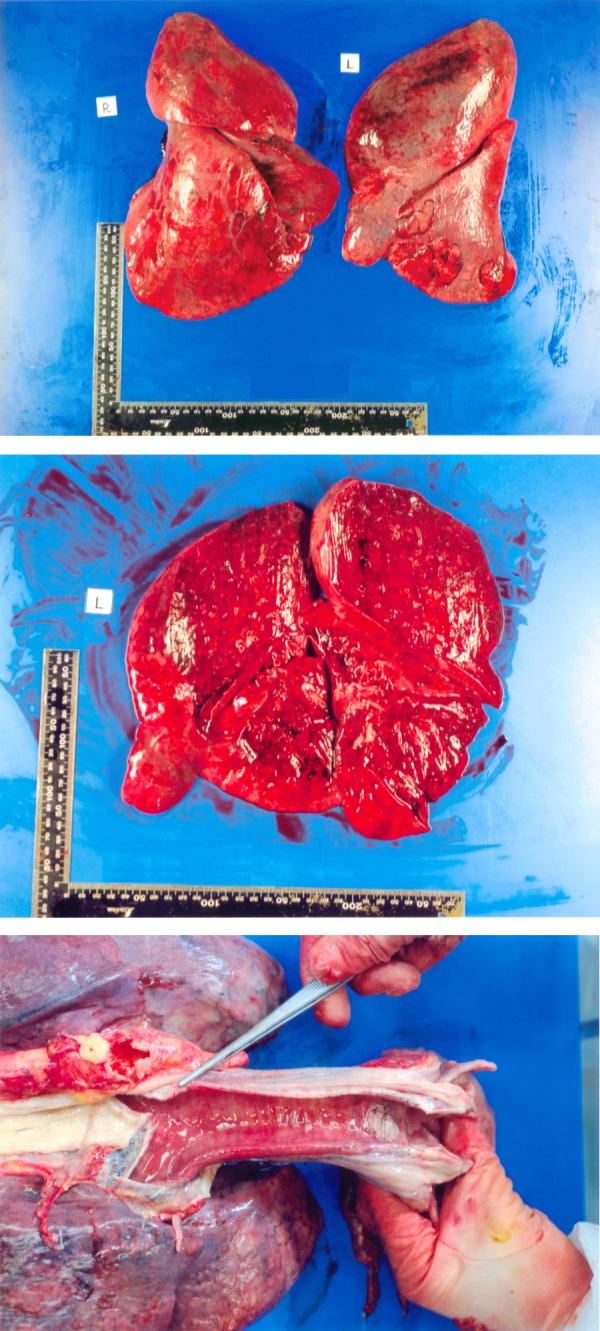
The autopsy revealed oedematous and haemorrhagic lungs. The weights of the right and left lungs were 882 and 1272 g, respectively (A and B). The tracheal mucosa was not injured (C).
Pathological examination revealed diffuse alveolar damage (DAD) over the entire lung (figure 5). Oedema, haemorrhage, proliferation of type II pneumocytes, thickening of the vascular wall and centrilobularly distributed squamous metaplasia were also observed. In some areas, the hyaline membranes lining the alveolar duct were visible. Notably, squamous metaplasia was more prominent in comparison to conventional acute interstitial pneumonia. Bronchial epithelia were sloughed off, which may be a postmortem change or as a result of the high concentration of oxygen that was needed several days before his death. In other lung fields, acute bacterial pneumonia was seen. The bacteria looked like bacilli, but the species could not be identified by H&E staining. Although this appearance was possibly a postmortem change, we assume that the bacterial pneumonia had occurred while he was alive. The lung was not emphysematous, and we could not find any cystic structures. The other organs showed unremarkable changes histopathologically.
Figure 5.
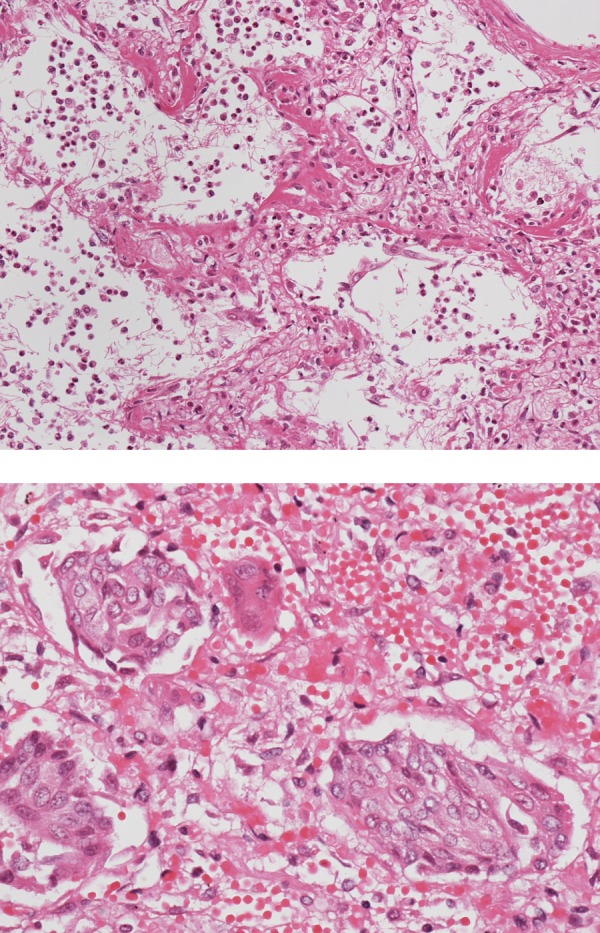
Pathological examination revealed diffuse alveolar damage in the entire lung. Oedema, haemorrhage, proliferation of type II pneumocytes, thickening of the vascular wall and centrilobularly distributed squamous metaplasia were also observed. In some areas, the hyaline membrane lining the alveolar duct was seen (A). Notably, squamous metaplasia was more prominent than that observed in conventional acute interstitial pneumonia (B).
Toxicological analysis
Toxicological analysis of PFAC in the blood and urine samples, that we collected on day 2 after admission, was carried out using a Shimadzu GC 14B gas chromatograph–mass spectrometry on a column (length 2 m, inside diameter 0.3 mm) containing 15% polyethylene glycol chromosorb w (AW/DMCS, 60–80 mesh), and with a programmed temperature of 80°C in the column, 150°C at the injection site and 150°C at the detector. The analytical samples were kept at 60°C for 15 min in a water bath, and 1 μL of headspace gas was injected. Nitrogen gas was used as a carrier gas with 100 kPa.
Some peaks were detected with retention times of 0.671, 1.452 and 3.017 min from the blood, and 1.446, 2.090, 2.677 and 3.302 min from the urine (figure 6). The highest peak from the blood sample was 1.452, and the highest one from the urine sample was 1.446. The peak near 4.58 in both graphs was an internal standard, n-propanol. We used allyl chloride as a reference for PFAC because we could not obtain PFAC owing to safety problems. The molecular structure of carbon and chloride in allyl chloride is the same as PFAC. Allyl chloride has all the hydrogen replaced by fluorine to form PFAC (figure 1). We obtained 1.21 min as the retention time of allyl chloride using our apparatus. Since we could not obtain pure PFAC, we could not measure its concentration.
Figure 6.
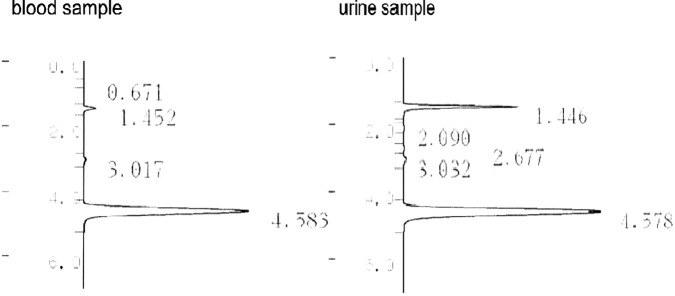
(Blood and urine samples) The highest peak of activity was observed at 1.452 in the blood sample and at 1.446 in the urine sample. The peak near 4.58 in both graphs was the internal standard, n-propanol.
Differential diagnosis
Acute chemical lung injury caused by 3-chloropentafluoropene(PFAC).
Treatment
We tried steroid pulse therapy and mechanical ventilation. However, the patient died.
Discussion
A man working at a chemical factory died of acute lung injury. We concluded that his death was due to acute chemical lung injury by PFAC based on interview, clinical course, findings of a thoracic CT, autopsy and our toxicological analysis. Of note, we showed the progression of lung injury by the toxic gas with CT and evaluated the histology of his lungs at autopsy.
PFAC has a boiling point at 7.6°C and a molecular weight of 166.48.1 According to CAS safety precautions, PFAC is highly flammable and is toxic by inhalation. Workers using PFAC must wear suitable protective gloves and clothing, and after handling, they must immediately take off all contaminated clothing. Although the precautions indicate high toxicity, the nature of the PFAC toxicity is not known. Furthermore, a method for identification of PFAC in exposed patient fluids was not previously established.
Only one unconfirmed fatal case of PFAC toxicity has been reported in 1981, and the clinical course and symptoms of that case are very similar to our case.5 According to the previous report, the patient came to the hospital for a cough, dyspnoea and fever. Infiltration of the lungs was found in the chest X-rays after hospitalisation, which did not respond to antibiotics. His respiratory condition deteriorated progressively and mechanical ventilation was needed. Finally, left-sided pneumothorax occurred and he died on day 16. The cause of his disease was unknown while he was alive. The investigation after his death discovered that he had washed a tank which had contained PFAC, so the cause of his death was concluded as lung injury by PFAC.
We tried to detect PFAC by gas chromatograph–mass spectrometry. We could see the highest peak at 1.452 min with the blood sample and at 1.446 min with urine sample. We suppose that the retention time is approximately 1.45 min. This retention time is consistent considering the retention time of 1.21 min of allyl chloride, which has a molecular structure similar to that of PFAC.
Our fatal case has been listed in the accidental case reports maintained by the Ministry of Health, Labour and Welfare (MHLW) in Japan. In another report from MHLW, a study on inhalation toxicity of PFAC in rats was performed by Stula.6 Stula found that the approximate lethal concentration of PFAC is 27 ppm, and that a significant effect of exposure to PFAC on the lung is observed at a concentration of 5.4 ppm. Rats exposed to 5.4 ppm had heavy lungs with acute inflammation of the alveolar tissue. This toxicity was very severe and similar to phosgene gas, which is well-known for its toxicity. The lethal concentration-time product of phosgene gas is 180–500 ppm min.7 8 Phosgene is also a fluorine compound. Accidental exposures to phosgene gas were reported, and the average clinical latent period lasts 6–15 h with a moderate phosgene dose.8 The clinical course is similar to our case. The mechanism of toxicity of PFAC may be similar to the other fluorine-containing compounds, including phosgene gas.
We observed the process of centrilobular ground-glass attenuation changing to consolidation with CT (figure 2). The consolidation was diffusely spread over the entire lung. Pathological examination revealed squamous metaplasia mainly existed around bronchi, and the structure of the lungs was discontinuous. On the other hand, the structure of the lungs was retained in areas of alveolar haemorrhage and oedema. Such pathological findings suggested that PFAC directly injured the bronchi, and subsequently, vascular hyperpermeability and accumulation of inflammatory cells occurred around the microwounds. Although squamous metaplasia is sometimes seen in DAD, it is not usually as prominent as in our case. Squamous metaplasia is probably one of the characteristic pathological findings of chemical lung injury by toxic gas9 because prominent squamous metaplasia was also seen in an autopsy case of a patient who had inhaled mustard gas.10 Although we diagnosed our patient while he was alive and treated him relatively quickly, we could not prevent his death. Protection against toxic gas is most important. We can perform neutralisation or detoxification therapy for some toxic compounds, but we can just provide oxygen administration or ventilation therapy for direct lung injury by toxic gas. In Japan, steroid pulse therapy was sometimes used in spite of no data on its benefit.
It is very difficult to diagnose chemical lung injury by toxic gas. Sudden death or rapid onset of symptoms due to high-dose toxic gas may be determined based on the environmental situation. However, slowly progressing symptoms due to accidental inhalation of low-dose toxic gas are difficult to detect. This report directly presented the clinical course of acute chemical lung injury caused by a highly toxic gas with CT imaging. When clinicians treat severe pneumonia similar to our case, they should ask the profession of the patient. We also defined a method to detect PFAC with gas chromatograph–mass spectrometry. Our toxicological analysis may be used as a reference for PFAC exposure in the future.
Learning points.
We could show the clinical course, findings of a thoracic CT, autopsy, pathological findings and toxicological analysis about PFAC.
Low-dose toxic gas injures alveolar tissue slowly. The early finding of thoracic CT looks like pleuritis.
When clinicians treat severe pneumonia similar to our case, they should ask the profession of the patient considering highly toxic gas.
When an autopsy of severe pneumonia shows prominent squamous metaplasia, death might have been caused by highly toxic gas.
The retention time of PFAC was presumed around 1.45 min by our toxicological analysis.
Footnotes
Contributors: SM contributed in the manuscript writing. SM and TT treated the patient. SM and KK made the pathological findings; and SM and KN made the toxicological analysis.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. http://www.cas-no.org/79-47-0 [Google Scholar]
- 2.Lawson IW, Smith MW, Stansfield N, et al. The reaction of 3-chloropentafluoropene with 1,4-dizabicyclo[2,2,2]octane on activated carbon. J Fluorine Chem 1992;58:170 [Google Scholar]
- 3.Kann GL, Smith S. Copolymers of 3-chloropentafluoropropylene with fluoro-olefins. Eur Polym J 1988;24:411–17 [Google Scholar]
- 4.Kvicala J, Paleta O. Reaction of (chlorodifluoromethyl) trifluorooxirane with nitrogen nucleophiles. J Fluorine Chem 1991;54:69 [Google Scholar]
- 5.Thun M, Kimbrough RD. Fatal chemical pneumonia from 1,1,2,3,3-pentafluoro-3-chloropropene in an unmarked gas tank. Clin Toxicol 1981;18:481–7 [DOI] [PubMed] [Google Scholar]
- 6.Stula EF. Abstracts of the 1984 Annual Meeting. Toxicologist 1984;4:66 [Google Scholar]
- 7.Wiliam E, Rinehart ScD, Theodore HSM. Concentration-time product (CT) as an expression of dose in sublethal exposures to phosgene. Am Ind Hyg Assoc J 1964;25:545–53 [DOI] [PubMed] [Google Scholar]
- 8.Lim SC, Yang JY, Jang AS, et al. Acute lung injury after phosgene inhalation. Korean J Intern Med 1996;11:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kevin OL, Mark RW. Practical pulmonary pathology: a diagnostic approach. 2nd edn London: Churchill Livingstone, 2011:117–18 [Google Scholar]
- 10.Tokuoka S, Hayashi Y, Nakagawa K, et al. Early cancer and related lesions in the bronchial epithelium in former workers of mustard gas factory. Acta Pathol Jpn 1986;36:533–42 [DOI] [PubMed] [Google Scholar]