Abstract
Temporal bone Schneiderian papilloma may present as a primary tumour originating from the middle ear and mastoid process, or an extension from sinonasal disease. Both forms are rare, this being only the 18th case of primary temporal bone Schneiderian papilloma described to date. Although the current patient has remained disease free after excision of the papilloma, the reported recurrence rate is high, comparable to sinonasal Schneiderian papilloma with extrasinus extension. Malignant progression of primary Schneiderian papillomas is significantly reduced as compared to Schneiderian papillomas that extend from the sinonasal tract into the temporal bone. A positive human papilloma virus status, as found in this case, is a common feature and prognostic factor of sinonasal Schneiderian papilloma but an infrequent finding in temporal bone disease. Owing to the high recurrence rate, the risk of malignant progression and the absence of reliable prognostic markers, stringent follow-up consisting of otoscopy, nasendoscopy and imaging is essential.
Background
Schneiderian-type papilloma (SP), also known as inverted papilloma, is a benign but locally aggressive tumour, most commonly involving the mucosa of the sinonasal tract. Localisation within the middle ear or mastoid process of the temporal bone is exceedingly rare. As the presenting symptoms and signs usually mimic common middle ear infections and thus are not very specific, the diagnosis is often delayed and is only made after histopathology of a biopsy or resection specimen.
There is discussion in the literature on whether temporal bone Schneiderian papilloma (TBSP) involves the same pathology as sinonasal SP.1 2 In TBSP, a degree of progesterone dependence has been suggested.3 Furthermore, higher recurrence and malignancy rates have been reported for TBSP as compared to sinonasal SP.1 Sinonasal SP is often associated with human papilloma virus (HPV) infections, and a positive HPV status seems a predictor for unfavourable outcomes. In TBSP, this association is not clear.4
Case presentation
A 74-year-old Caucasian woman was referred to our clinic for a conductive hearing loss and recurrent right-sided middle ear infections presenting as episodes of painless otorrhoea. The symptoms had started 2 years before the presentation at our clinic and tympanostomy and various eardrops had not resolved the problem.
Otoscopy of the right ear revealed a livid-coloured mass behind an intact eardrum. The facial nerve function was intact and the examination of the nose, including nasendoscopy, the throat and neck did not show any abnormalities.
Investigations
Pure tone audiometry showed a mixed-type hearing loss of around 50 dB on the right side, with a conductive component of 30 dB. High-resolution CT (HRCT) of the petrous bone revealed an opacified right mesotympanum, epitympanum and mastoid. No signs of bone erosion were seen, and the ossicular chain, fallopian canal and labyrinth were intact. The left side showed a normal middle ear and mastoid (figure 1).
Figure 1.
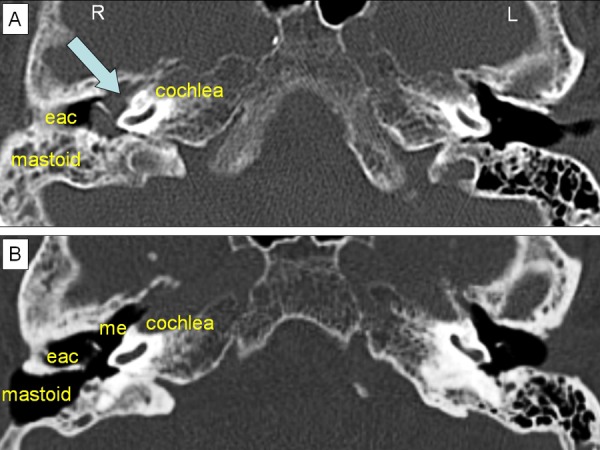
Preoperative and postoperative axial high-resolution CT images of the temporal bone. (A) The preoperative image shows complete opacification of the middle ear and partial opacification of the mastoid process (mastoid), indicating a soft tissue mass (blue arrow). (B) The postoperative image shows the postoperative exenteration of the mastoid cells and the open connection with the middle ear (me) through an opened facial recess. The complete absence of opacification in the mastoid process and middle ear indicates no residual disease. eac, external ear canal.
Differential diagnosis
The differential diagnosis after otoscopy, audiometry and HRCT imaging of the temporal bone includes a wide variety of space occupying lesions in the middle ear. Although otoscopy did not show signs of cholesteatoma, it is relatively prevalent and could not be fully excluded. Other possibilities are all uncommon and include benign neoplasms such as paraganglioma, papilloma and carcinoid tumour, several types of primary malignancy (including squamous cell carcinoma, adenocarcinoma, adenoid cystic carcinoma and sarcoma) and metastasis.
Treatment
A transcanal middle ear inspection was performed, which showed an intact tympanic membrane and livid-coloured, polypoid tissue in the middle ear without signs of ossicular destruction or keratosis. A biopsy was obtained, and histopathology showed an SP without features indicative of malignancy (figure 2). Subsequently, a combined transmastoidal and transcanal approach of the middle ear was performed, with exenteration of the mastoid cells and opening of the antrum and facial recess (figure 1). The ossicular chain was briefly disconnected at the incudostapedial joint to allow better access and reattached with tissue glue. The polypoid tissue was found to be confined to the middle ear and could be completely excised.
Figure 2.
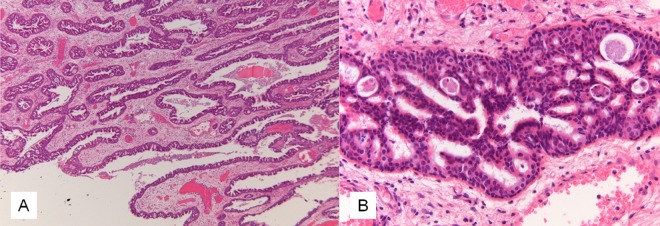
Histopathology of the Schneiderian papilloma in the middle ear. (A) Low magnification showing aggregates of hyperplastic respiratory epithelium with invagination throughout the stroma. (B) Detail showing small cysts in the epithelial lining containing cellular debris and neutrophils.
Outcome and follow-up
Histopathology showed an exophytic type SP. GP5+/6+ PCR with enzyme immunoassay for HPV proved positive for low risk-HPV, but negative for high risk-HPV. Progesterone and oestrogen receptor immunohistochemistry was negative. Less than 1% of the cells showed expression of the proliferative markers TP53 or MIB1.
The postoperative follow-up consisted of regular clinical investigation including otoscopy, nasendoscopy, audiometry, HRCT and MRI. Postoperative audiometry showed an improvement in conductive hearing of 25 dB in the right ear. HRCT and MRI 1, 3 and 5 years postoperatively showed no signs of recurrence or sinonasal involvement. The patient is still disease free at 5 years follow-up.
Discussion
To date, 33 patients with TBSP have been reported (table 1). In only 18 patients, including the current case, did the tumour arise as a primary, isolated lesion in the temporal bone, representing true primary TBSP. In 14 cases, the temporal bone localisation represented an extension from primary sinonasal SP, and in one case, sinonasal involvement is not clearly described.
Table 1.
Schneiderian papilloma of the temporal bone
| Author | Year | Sex | Age | Primary TBSP | HPV | TP53 | MIB1 | P/O | Recurrence | Association with carcinoma in temporal bone | FU |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stone et al | 1987 | M | 55 | No | No | Squamous cell carcinoma in mastoid | NED | ||||
| Altug et al | 1989 | F | 64 | Yes | No | Carcinoma in mastoid | AWD | ||||
| Kaddour et al | 1992 | F | No | No | No | No | No data | ||||
| Roberts et al | 1993 | F | 19 | Yes | −(ISH) | No | No | NED 6 months | |||
| Bold et al | 1995 | F | 29 | No data | No data | No data | No data | ||||
| Seshul et al | 1995 | F | 31 | No | −(method unknown) | P+, O− | No | Squamous cell carcinoma in nasal cavity, external auditory canal, mastoid | AWD | ||
| Wenig | 1996 | F | 31 | Yes | −(ISH) | Yes, 7 in 15 years | No | NED 60 months | |||
| F | 56 | Yes | −(ISH) | Yes, 2 in 2 years | No | NED 144 months | |||||
| F | 19 | Yes | −(ISH) | No | No | NED 120 months | |||||
| F | 57 | Yes | −(ISH) | Yes, 2 in 6 years | No | NED 84 months | |||||
| F | Yes | −(ISH) | Yes | No | NED 6 months | ||||||
| Jones et al | 1998 | F | 35 | No | +(PCR) | No | Carcinoma in mastoid | NED 14 months | |||
| Vural et al | 1999 | F | 44 | No | No | No | NED 8 years | ||||
| Chhetri et al | 2001 | M | 26 | Yes | Yes, after 6 months | No | NED 14 months | ||||
| de Filippis et al | 2002 | M | 58 | Yes | −(ISH) | No | No | NED 3 months | |||
| Pou et al | 2002 | M | 81 | No | Yes, after 6 months | Squamous cell carcinoma in temporal bone (carcinoma in situ in primary lesion) | DOD 36 months | ||||
| M | 54 | No | No | Squamous cell carcinoma in temporal bone and nasal cavity | NED 11 months | ||||||
| Blandamura et al | 2003 | M | 54 | Yes | 44% | 2% | P+, O− | No data | No data | No data | |
| M | 58 | Yes | 5% | 13% | P−, O− | No | No | NED 14 months | |||
| Cahali et al | 2005 | M | 72 | Yes | −(serology) | No data | No data | No data | |||
| Mazlina et al | 2006 | M | 54 | No | No | Poorly differentiated carcinoma in mastoid and lymph node | No data | ||||
| de Menezes Santos Torres et al | 2007 | F | 27 | Yes | No | No | NED 6 months | ||||
| Jung et al | 2009 | M | 9 | Yes | No | No | NED 4 months | ||||
| Acevedo-Henao et al | 2010 | M | 63 | No | Yes, 4 in 5 years | Epidermoid carcinoma in temporal bone | DOD 6 years | ||||
| Inoue et al | 2010 | F | 53 | Yes | −(PCR) | No | No | NED 11 years | |||
| Kainuma et al | 2011 | M | 65 | No | −(immunostaining) | Yes, after 2 months | No | NED 10 months | |||
| Zhou et al | 2011 | M | 52 | Yes | No | Carcinoma in situ in primary sinonasal and temporal lesion | NED 8 months | ||||
| Shen et al | 2011 | M | 56 | No | No | Papillary squamous cell carcinoma nasal cavity | NED 6 months | ||||
| Ali et al | 2011 | F | 42 | Yes | No data | No data | No data | ||||
| Uchida et al | 2011 | F | 52 | No | −HR,+LR(method unknown) | Yes, contralateral ear | No data | No data | |||
| Dingle et al | 2012 | M | 52 | No | −(method unknown) | Yes, after 2 and 6 months (right) | Squamous cell carcinoma in right temporal bone | AWD | |||
| Mitchell et al | 2012 | F | 69 | No | No | Areas of carcinoma in situ in primary sinonasal and temporal lesion | NED 3 years | ||||
| Current case | 2013 | F | 74 | Yes | −HR (PCR), +LR (PCR) | <1% | <1% | P−,O− | No | No | NED 5 years |
AWD, alive with disease; DOD, dead of disease; F, female; FU, follow-up; HPV, human papilloma virus status; HR, high risk HPV serotype; ISH, in situ hybridisation; LR, low risk HPV serotype; M, male; MIB1, Ki-67 marker; NED, no evidence of disease; P/O, progesterone and oestrogen; TBSP, temporal bone Schneiderian papilloma; TP53, tumour protein 53.
While sinonasal SP occurs predominantly in men, our review of the literature shows a slight female predominance of 55% for TBSP (table 1). Adequate data on follow-up were available for 28 of 33 reviewed patients with TBSP, 15 primary TBSP and 13 TBSP with sinonasal involvement. There is no significant difference in the recurrence rate of primary TBSP (5/15; 33%) and of TBSP with sinonasal involvement (4/13; 31%; table 1). This high recurrence rate is comparable to the 35% recurrence rate of primary sinonasal SP with extrasinus extension (group C sinonasal SP according to the prognostic staging system of Cannady et al).5 As in sinonasal SP, the recurrence rate of TBSP has been reported to depend on the type of intervention: 67% after limited excisions and 10% after extensive excisions.1
Twelve patients with TBSP were diagnosed with carcinoma of the temporal bone (43%). This exceeds the risk of malignancy in primary sinonasal SP (5–13%).4 6 Remarkably however, our review shows that the risk of malignant transformation is significantly higher for TBSP with sinonasal involvement (10/13; 77%), than for primary TBSP confined to the temporal bone (2/15; 13%; p=0.001).
Several putative prognostic markers have been evaluated in TBSP, including HPV status, sex hormones, TP53 and MIB1 immunohistochemistry.
HPV status has been reported in only 15 TBSP cases. Thus far, three TBSP cases with sinonasal involvement tested HPV positive (20%). The current case represents the first HPV positive primary TBSP (low-risk HPV type). HPV therefore seems to play a considerably less prominent role in TBSP than in sinonasal SP, where HPV positivity is reported in 76% of patients and is associated with recurrences and malignant course of the disease.2 4
Sex steroid hormones have been hypothesised to influence TBSP proliferation, because a rapid tumour growth has been observed during pregnancy.3 Until now, only four patients have been tested for oestrogen and progesterone receptor status, including the current case. Oestrogen receptor staining was negative in all tested patients. Progesterone receptor negative TBSP was found in two of four patients, including the current case. Both patients have remained disease free during follow-up. Two progesterone receptor positive patients with TBSP had concurrent sinonasal involvement. One developed a temporal bone carcinoma and the other was lost to follow-up.
TP53 and MIB1 have been investigated as markers for the progression to malignancy in only three patients with TBSP. The reported results vary, with a reported TP53 expression ranging from less than 1–44%, and MIB1 expression ranging from less than 1–13% (table 1).
The limited number of TBSPs that have been tested for prognostic markers, especially for sex hormone receptors TP53 or MIB1, precludes definite conclusions on their value.
Learning points.
Temporal bone localisation of a Schneiderian papilloma may represent a primary tumour or an extension from the sinonasal tract; both are rare.
The clinical presentation and radiology of temporal bone Schneiderian papilloma are not very specific and the diagnosis can only be reliably made after histopathology of a biopsy or resection specimen.
The recurrence rate after surgery, the therapy of choice, is high (32%), and seems to be higher when limited excisions have been performed.
There is a risk of malignancy developing from Schneiderian papilloma of the temporal bone, however true primary temporal bone papillomas seem to confer a much lower risk than papillomas with sinonasal involvement.
Owing to the risk of recurrence, the risk of progression to malignancy, and the absence of reliable prognostic markers, strict postoperative follow-up is mandatory and should consist of regular otoscopy, nasendoscopy and imaging.
Acknowledgments
The authors would like to thank Dr Conrad F Smit for critically revising the manuscript.
Footnotes
Contributors: LvdP acquired data, performed the analysis and drafted the manuscript. EB acquired data, performed additional investigations and critically revised the manuscript. PM acquired data, assisted in the interpretation and critically revised the manuscript. EFH initialised the case report, performed the analysis and interpretation of the data, and helped in drafting the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.de Filippis C, Marioni G, Tregnaghi A, et al. Primary inverted papilloma of the middle ear and mastoid. Otol Neurotol 2002;23:555–9 [DOI] [PubMed] [Google Scholar]
- 2.Jones ME, Wackym PA, Said-Al-Naief N, et al. Clinical and molecular pathology of aggressive Schneiderian papilloma involving the temporal bone. Head Neck 1998;20:83–8 [DOI] [PubMed] [Google Scholar]
- 3.Blandamura S, Marioni G, de Filippis C, et al. Temporal bone and sinonasal inverted papilloma: the same pathological entity? Arch Otolaryngol Head Neck Surg 2003;129:553–6 [DOI] [PubMed] [Google Scholar]
- 4.Wassef SN, Batra PS, Barnett S. Skull base inverted papilloma: a comprehensive review. ISRN Surg 2012;2012:175903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannady SB, Batra PS, Sautter NB, et al. New staging system for sinonasal inverted papilloma in the endoscopic era. Laryngoscope 2007;117:1283–7 [DOI] [PubMed] [Google Scholar]
- 6.Chhetri DK, Gajjar NA, Bhuta S, et al. Pathology forum. Quiz case 2. Schneiderian-type papilloma of the middle ear. Arch Otolaryngol Head Neck Surg 2001;127:79, 80–2 [PubMed] [Google Scholar]


