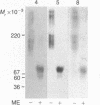
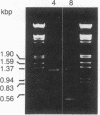
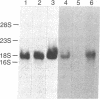
Abstract
Messenger RNA, prepared from fetal bovine testicular tissue, was used to construct a cDNA library in lambda gt11 phage. The library was screened with an antibody probe directed against bovine anti-Müllerian hormone and three positive clones were isolated. Cross-hybridizing cDNA inserts carried by clones 4 and 5 (1.2 and 0.08 kilobases long, respectively) code for a fragment of authentic anti-Müllerian hormone, as shown by the ability of the anti-epitope antibodies eluted from fusion protein 4 to bind strongly to anti-Müllerian hormone on immunoblots and by the capacity of anti-epitope antibodies 4 and 5 to precipitate radioiodinated bovine anti-Müllerian hormone. A probe prepared from insert 4 hybridizes with an mRNA present only in tissues that are known producers of anti-Müllerian hormone, such as the fetal testis and adult ovarian follicles. The amount of specific mRNA in tissues of males and females is related to the rate of their anti-Müllerian hormone production. The 2.1-kilobase size of this mRNA species is large enough to code for the Mr 62,000 anti-Müllerian hormone polypeptide chain. Insert 4 also hybridizes with an mRNA of similar size in human and rat fetal testicular tissue. The third isolated clone, clone 8, which does not cross-hybridize with the others, carries a cDNA insert coding for a ubiquitous protein, smaller than anti-Müllerian hormone, with which it apparently shares an epitope.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Blanchard M. G., Josso N. Source of the anti-Müllerian hormone synthesized by the fetal testis: Müllerian-inhibiting activity of fetal bovine Sertoli cells in tissue culture. Pediatr Res. 1974 Dec;8(12):968–971. doi: 10.1203/00006450-197412000-00011. [DOI] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Govindan M. V., Devic M., Green S., Gronemeyer H., Chambon P. Cloning of the human glucocorticoid receptor cDNA. Nucleic Acids Res. 1985 Dec 9;13(23):8293–8304. doi: 10.1093/nar/13.23.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Shima H., Hayashi K., Trelstad R. L., Donahoe P. K. Immunocytochemical localization of Mullerian inhibiting substance in the rough endoplasmic reticulum and Golgi apparatus in Sertoli cells of the neonatal calf testis using a monoclonal antibody. J Histochem Cytochem. 1984 Jun;32(6):649–654. doi: 10.1177/32.6.6373916. [DOI] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Daegelen D., Dreyfus J. C. Cell-free translation of messenger RNAs from adult and fetal human muscle. Characterization of neosynthesized glycogen phosphorylase, phosphofructokinase and glucose phosphate isomerase. Eur J Biochem. 1981 May;116(1):7–12. doi: 10.1111/j.1432-1033.1981.tb05293.x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Picard J. Y., Goulut C., Bourrillon R., Josso N. Biochemical analysis of bovine testicular anti-Müllerian hormone. FEBS Lett. 1986 Jan 20;195(1-2):73–76. doi: 10.1016/0014-5793(86)80133-8. [DOI] [PubMed] [Google Scholar]
- Picard J. Y., Josso N. Purification of testicular anti-Müllerian hormone allowing direct visualization of the pure glycoprotein and determination of yield and purification factor. Mol Cell Endocrinol. 1984 Jan;34(1):23–29. doi: 10.1016/0303-7207(84)90155-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Josso N. Localization of anti-Müllerian hormone in the rough endoplasmic reticulum of the developing bovine sertoli cell using immunocytochemistry with a monoclonal antibody. Endocrinology. 1982 Nov;111(5):1562–1567. doi: 10.1210/endo-111-5-1562. [DOI] [PubMed] [Google Scholar]
- Vigier B., Legeai L., Picard J. Y., Josso N. A sensitive radioimmunoassay for bovine anti-Müllerian hormone, allowing its detection in male and freemartin fetal serum. Endocrinology. 1982 Oct;111(4):1409–1411. doi: 10.1210/endo-111-4-1409. [DOI] [PubMed] [Google Scholar]
- Vigier B., Picard J. Y., Campargue J., Forest M. G., Heyman Y., Josso N. Secretion of anti-Müllerian hormone by immature bovine Sertoli cells in primary culture, studied by a competition-type radioimmunoassay: lack of modulation by either FSH or testosterone. Mol Cell Endocrinol. 1985 Dec;43(2-3):141–150. doi: 10.1016/0303-7207(85)90077-2. [DOI] [PubMed] [Google Scholar]
- Vigier B., Picard J. Y., Tran D., Legeai L., Josso N. Production of anti-Müllerian hormone: another homology between Sertoli and granulosa cells. Endocrinology. 1984 Apr;114(4):1315–1320. doi: 10.1210/endo-114-4-1315. [DOI] [PubMed] [Google Scholar]
- Vigier B., Tran D., du Mesnil du Buisson F., Heyman Y., Josso N. Use of monoclonal antibody techniques to study the ontogeny of bovine anti-Müllerian hormone. J Reprod Fertil. 1983 Sep;69(1):207–214. doi: 10.1530/jrf.0.0690207. [DOI] [PubMed] [Google Scholar]
- Walter P., Green S., Greene G., Krust A., Bornert J. M., Jeltsch J. M., Staub A., Jensen E., Scrace G., Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- de Keyzer Y., Bertagna X., Lenne F., Girard F., Luton J. P., Kahn A. Altered proopiomelanocortin gene expression in adrenocorticotropin-producing nonpituitary tumors. Comparative studies with corticotropic adenomas and normal pituitaries. J Clin Invest. 1985 Nov;76(5):1892–1898. doi: 10.1172/JCI112184. [DOI] [PMC free article] [PubMed] [Google Scholar]