Abstract
Infective endocarditis (IE) is more common in left heart chambers in the general population. Right-sided IE is increased especially in intravenous drug misusers. However, involvement of both right and left heart chambers is extremely rare in these patients. In this study, we present the case of a 26-year-old male intravenous drug misuser, hospitalised with Staphylococcal tricuspid valve IE complicated with pulmonary septic embolism. After 10 days, an echocardiogram revealed vegetation on the mitral valve in addition to unchanged tricuspid valve vegetation. IE can be a rapidly progressive disease despite the medical treatment in intravenous drug misusers. Generally, the right-sided IE is common in intravenous drug misusers; however, as in our case, it may involve left heart chambers as well. Therefore, in the right-sided IE cases, a serial echocardiographic examination of both heart chambers is strictly recommended.
Background
Infective endocarditis (IE) is a disease of the cardiac endothelial surface, caused by several microbial pathogens. Despite the new diagnostic and treatment modalities, prognosis is still poor and mortality is high. Neither incidence nor the mortality rate of IE is decreased for 30 years. In general population, IE is more common in the left heart chambers and right-sided IE is increased especially in intravenous drug misusers. In IE patients with a history of intravenous drug misuse, 86% had right-sided and 14% had left-sided involvement.1 However, both right and left heart chamber involvement are extremely rare. In this paper, we aimed to present a rare case of IE in an intravenous drug misuser involving both cardiac chambers.
Case presentation
A 26-year-old man presented to emergency service with symptoms of continuing dyspnoea, chest pain, cough, malaise for 4 weeks and fever for a week. Body temperature was 38.7°C, and he had sinusal tachycardia with a pulse rate of 110 bpm. A physical examination revealed crepitating rales heard over bilateral basal and middle lung fields. Among laboratory findings white blood count and C reactive protein were elevated (19 800 K/µL and 169 mg/dL, respectively). A chest X-ray revealed infiltrative changes in both lung fields and the patient was hospitalised to the Chest Diseases Clinic with the diagnosis of pneumonia. Vancomycin treatment was initiated. A thoracic CT revealed widespread, peripherally located irregular consolidated areas with air bronchograms which tend to coalesce and are more prominent on the basal segments of the lower lobe of the right lung (figure 1). Detailed medical history showed that the patient was on intravenous drug misuse for almost a year. After this stage, the patient was referred to cardiology clinic with the diagnosis of IE. On auscultation, a grade 3/6 systolic murmur was heard over the left-lower sternal border and mezocardiac area. A transthoracic echocardiography showed a mobile mass on the tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of the anterior leaflet of the tricuspid valve (figure 2A, B and videos 1–4). Estimated pulmonary arterial pressure was 65 mm Hg. There was no vegetation observed in the left heart structures. The patient was transferred to the cardiology clinic with the diagnosis of IE. Three sets of blood cultures were taken. The combination of vancomycin and gentamicin was initiated, which is the empirical treatment to Staphylococcus aureus the most common cause of IE in intravenous drug misusers. After a transthoracic echocardiogram, a transoesophageal echocardiography was recommended; however, the patient did not consent to the procedure. The patient showed no evidence of peripheral stigmatas of IE. Blood culture results yielded isolate of vancomycin and gentamicin-sensitive S aureus. We continued with the available medical treatment as it complied with antibiogram. A control echocardiogram on the 10th day revealed a vegetation on the mitral valve in addition to unchanged tricuspid valve vegetation (figure 3A, B and videos 5 and 6). The patient, who was recommended surgical treatment because of the rapid progression of vegetation and severe valvular regurgitations, did not consent this treatment. On the 20th day of follow-up, the patient wanted to be discharged against medical advice.
Figure 1.
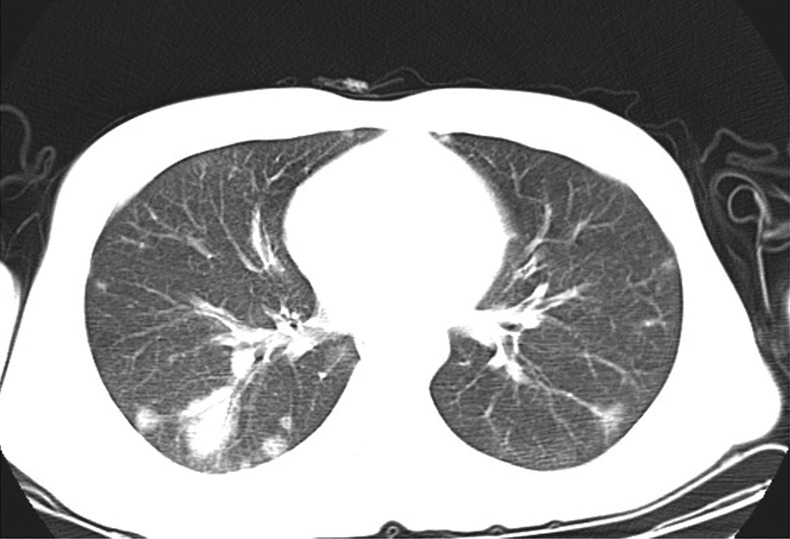
Thoracic CT showing widespread, peripherally located irregular consolidated areas with air bronchograms which tend to coalesce and more prominent on the basal segments of the lower lobe of the right lung.
Figure 2.
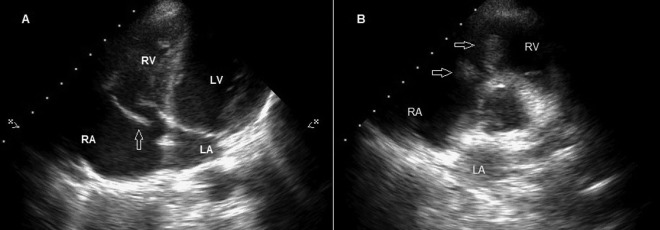
(A) Transthoracic echocardiography shows chordal rupture of anterior leaflet of tricuspid valve on the apical four-chamber view (arrow). (B) Transthoracic echocardiography showing a mobile mass on tricuspid valve compatible with vegetation on the short axis view (arrows).
Figure 3.
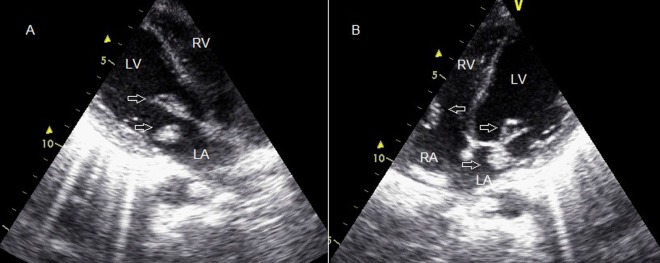
(A) Control echocardiogram at the 10th day showing vegetation on mitral valve (arrows). (B) Control echocardiogram at the 10th day showing vegetation on mitral valve in addition to unchanged tricuspid valve vegetation on the apical four-chamber view (arrows).
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Control echocardiogram at the 10th day revealed a vegetation on mitral valve in addition to unchanged tricuspid valve vegetation.
Control echocardiogram at the 10th day revealed a vegetation on mitral valve in addition to unchanged tricuspid valve vegetation.
Discussion
Intravenous drug misusers have an increased risk for IE.2 The exact incidence of IE in intravenous drug misusers is not known; it is estimated to be 1.5–20 for every 1000 intravenous drug misusers in a year. Right heart involvement is more common in intravenous drug users.3 The tricuspid valve is the most commonly affected valve followed by mitral and aortic valves. Both cardiac chamber involvements are extremely rare. It is important to show multivalve involvement of patients with suspected IE. Two or more valve involvement has higher morbidity and mortality than single valve involvement, and early surgery may be required for the treatment of the complications in these patients.4
S aureus is the most common pathogen in intravenous drug misusers with IE.5 In a study, in which Carozza et al6 investigated intravenous drug misusers, it was stated that the most common causative agent was S aureus in the right-sided endocarditis as in our case while Streptococcus viridans and S aureus in the left-sided endocarditis. IE can show rapid progression in intravenous drug misusers. In a case of Piran et al7 both cardiac chamber and four valve involvement was reported in a patient with a history of intravenous drug misuse. In that case, first, the patient was given medical treatment due to increased perioperative risk caused by multiple valve involvement. However, in the follow-up of the patient, severe mitral regurgitation developed and replacement of all four valves was the treatment of choice. This shows the importance of surgery in early stages of IE in order to prevent rapid progression.
The expected benefits of antibiotic treatment given before surgery in patients with complicated IE cannot be more beneficial than the time lost due to delays in the operation. Moreover, patients who underwent early surgery with insufficient period of antibiotic administration did not increase the risk of severe postoperative complications.8 Additionally, surgery may improve survival.8 In our case, an initially right-sided vegetation spread to the left side. It was thought that the reason for the rapid progression of vegetation from the right cardiac chambers to the left cavities was that the patient was immunosuppressed due to intravenous drug misuse; as well as the patient observed with high pathogenicity of S aureus.
Learning points.
Infective endocarditis (IE) can be a rapidly progressive disease despite the medical treatment in intravenous drug misusers.
Generally the right-sided IE is common in intravenous drug misusers; however, as in our case, it may involve left heart chambers as well.
In right-sided IE, serial echocardiographic examination of both heart chambers is strictly recommended.
Footnotes
Contributors: MO participated in clinical following, approval of case. SE participated in echocardiographic examination; FB participated in design and interpretation; VD participated in editing the language of manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Moss R, Munt B. Injection drug use and right sided endocarditis. Heart 2003;89:577–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiq S, Missri J, Silverman I. Endocarditis in an urban hospital in the 1990s. Arch Intern Med 1996;156:2454–8 [PubMed] [Google Scholar]
- 3.Crane LR, Levine DP, Zervos MJ, et al. Bacteremia in narcotic addicts at the Detroit Medical Centre. Microbiology, epidemiology, risk factors and empiric therapy. Rev Int Dis 1986;8:364–73 [DOI] [PubMed] [Google Scholar]
- 4.Chambers HF, Korzeniowski OM, Sande MA. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine 1983;62:170–7 [PubMed] [Google Scholar]
- 5.Miro JM, Del Rio A, Mestres CA. Infective endocarditis and cardiac surgery in intravenous drug abusers and HIV-1 infected patients. Cardiol Clin 2003;21:167–84 [DOI] [PubMed] [Google Scholar]
- 6.Carozza A, Romano G, De Feo M. Infective endocarditis in intravenous drug users: prevalence of left heart involvement and changing microbiologic profile . Paper presented at: 7th International Symposium on Modern Concepts in Endocarditis and Cardiovascular Infections; 26–28 June 2003, Chamonix, France. abstract 53 [Google Scholar]
- 7.Piran S, Rampersad P, Kagal D, et al. Extensive fulminant multivalvular infective endocarditis. J Am Coll Cardiol Img 2009;2:787–9 [DOI] [PubMed] [Google Scholar]
- 8.Thuny F, Beurtheret S, Mancini J, et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J 2011;32:2027–33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Transthoracic echocardiography shows a mobile mass on tricuspid valve compatible with vegetation, severe eccentric tricuspid regurgitation and chordal rupture of anterior leaflet of tricuspid valve.
Control echocardiogram at the 10th day revealed a vegetation on mitral valve in addition to unchanged tricuspid valve vegetation.
Control echocardiogram at the 10th day revealed a vegetation on mitral valve in addition to unchanged tricuspid valve vegetation.


