Abstract
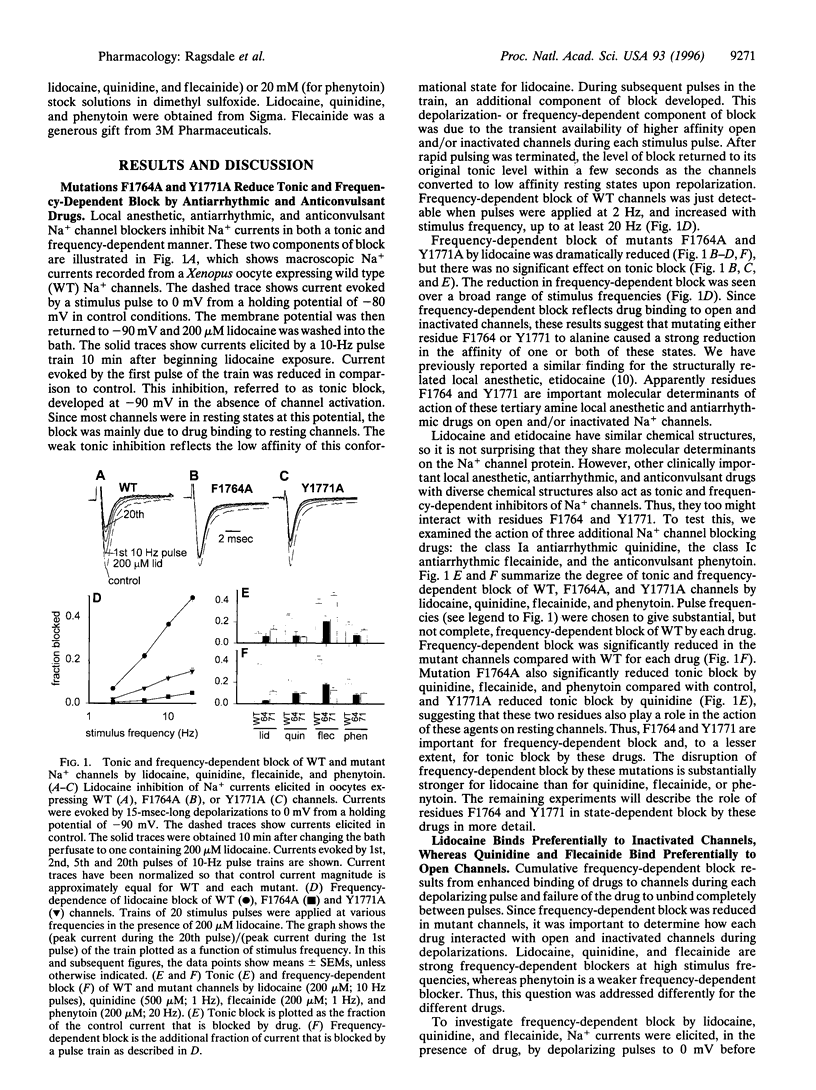
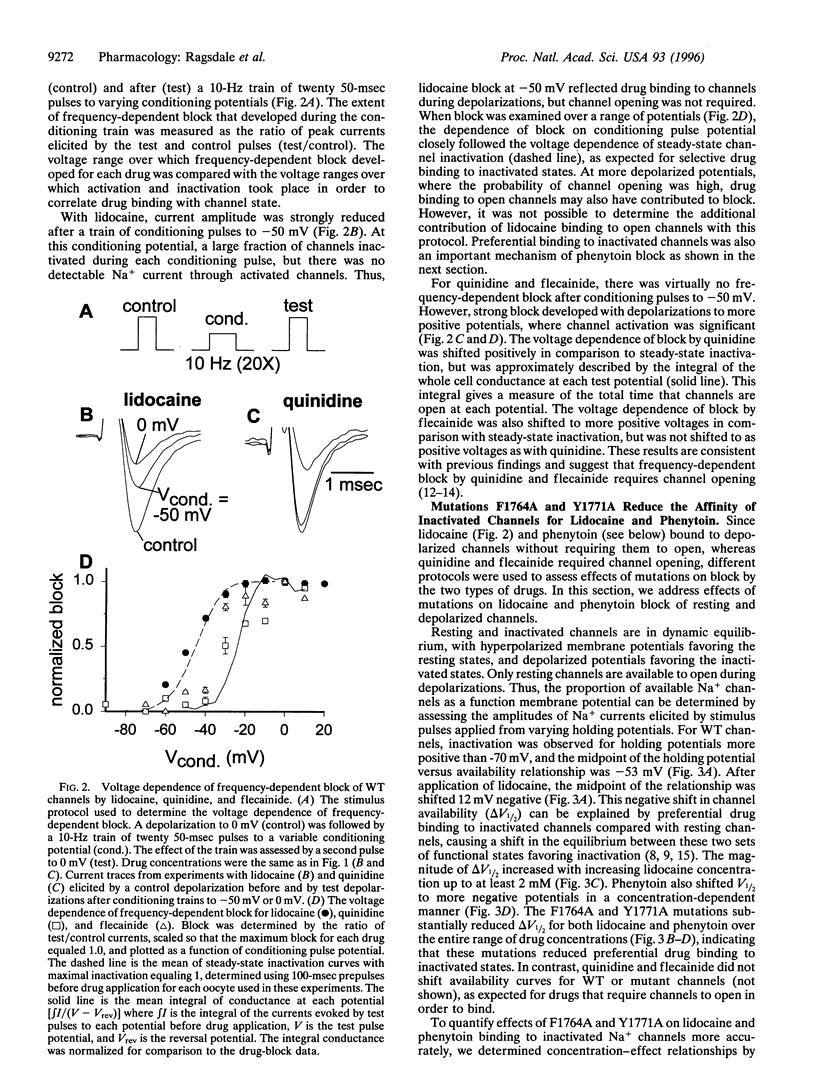
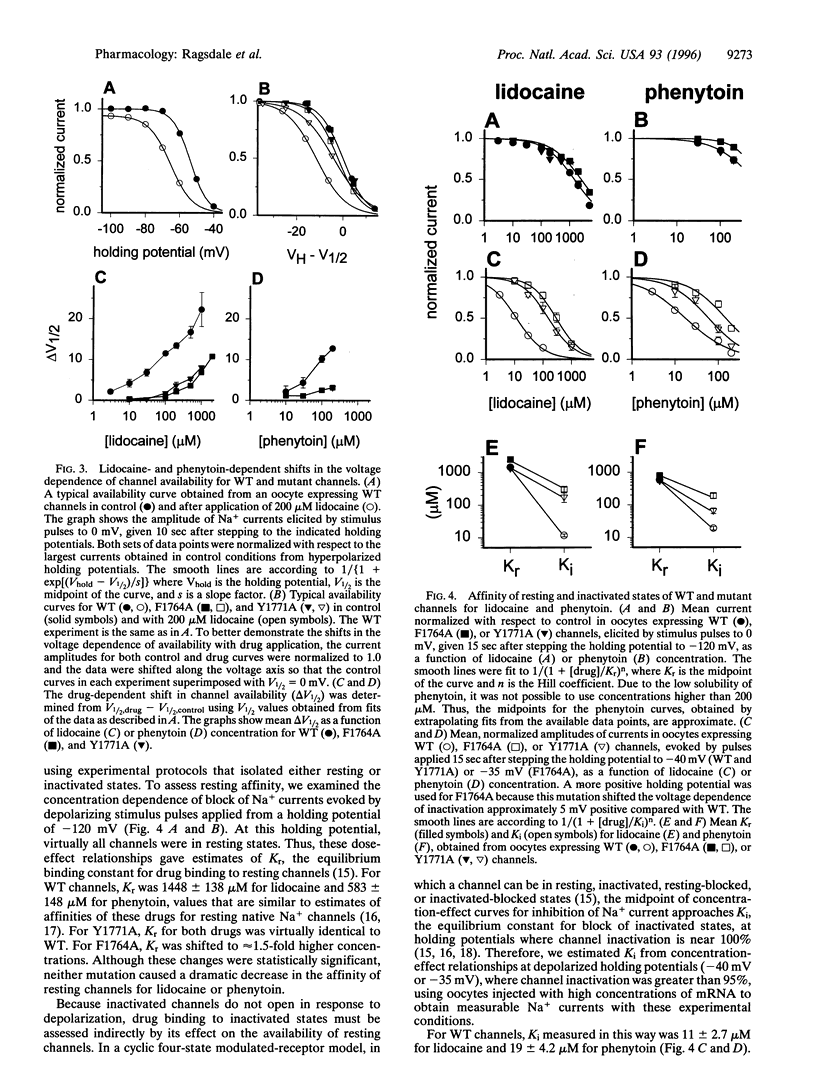
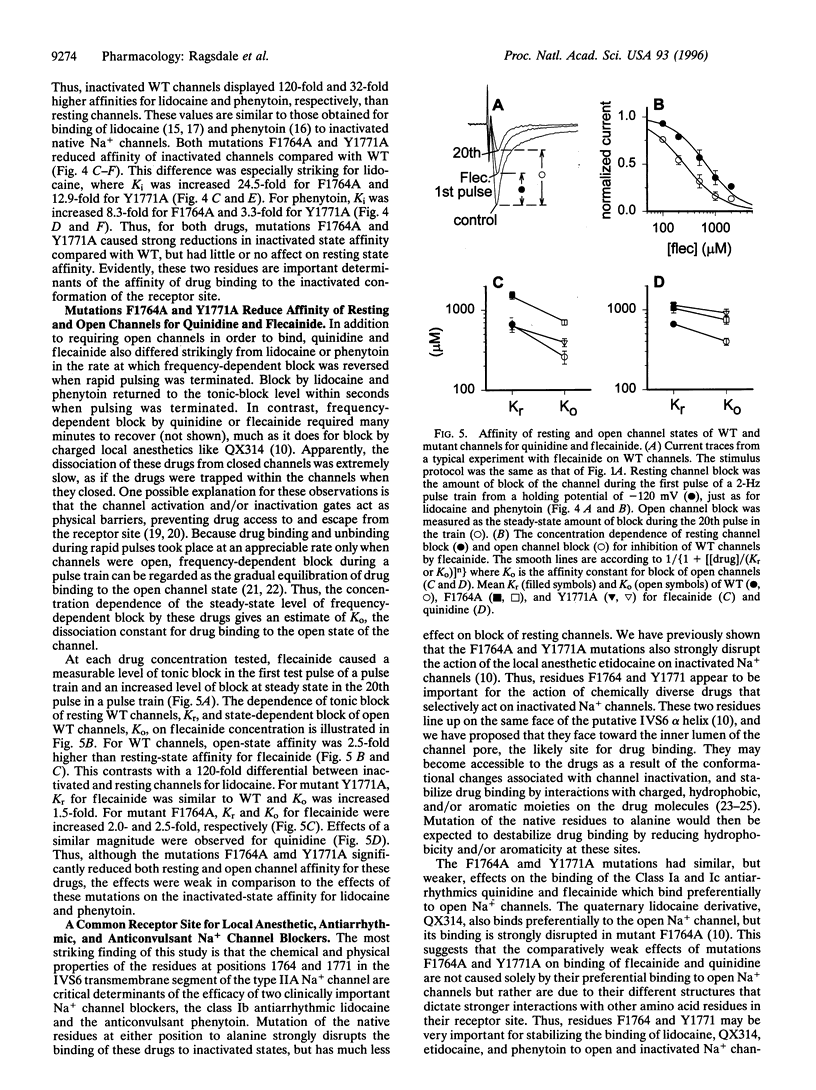
Voltage-gated Na+ channels are the molecular targets of local anesthetics, class I antiarrhythmic drugs, and some anticonvulsants. These chemically diverse drugs inhibit Na+ channels with complex voltage- and frequency-dependent properties that reflect preferential drug binding to open and inactivated channel states. The site-directed mutations F1764A and Y1771A in transmembrane segment IVS6 of type IIA Na+ channel alpha subunits dramatically reduce the affinity of inactivated channels for the local anesthetic etidocaine. In this study, we show that these mutations also greatly reduce the sensitivity of Na+ channels to state-dependent block by the class Ib antiarrhythmic drug lidocaine and the anticonvulsant phenytoin and, to a lesser extent, reduce the sensitivity to block by the class Ia and Ic antiarrhythmic drugs quinidine and flecainide. For lidocaine and phenytoin, which bind preferentially to inactivated Na+ channels, the mutation F1764A reduced the affinity for binding to the inactivated state 24.5-fold and 8.3-fold, respectively, while Y1771A had smaller effects. For quinidine and flecainide, which bind preferentially to the open Na+ channels, the mutations F1764A and Y1771A reduced the affinity for binding to the open state 2- to 3-fold. Thus, F1764 and Y1771 are common molecular determinants of state-dependent binding of diverse drugs including lidocaine, phenytoin, flecainide, and quinidine, suggesting that these drugs interact with a common receptor site. However, the different magnitude of the effects of these mutations on binding of the individual drugs indicates that they interact in an overlapping, but nonidentical, manner with a common receptor site. These results further define the contributions of F1764 and Y1771 to a complex drug receptor site in the pore of Na+ channels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anno T., Hondeghem L. M. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage dependence of recovery. Circ Res. 1990 Mar;66(3):789–803. doi: 10.1161/01.res.66.3.789. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. B., Valenzuela C., Chen L. Q., Kallen R. G. On the molecular nature of the lidocaine receptor of cardiac Na+ channels. Modification of block by alterations in the alpha-subunit III-IV interdomain. Circ Res. 1995 Sep;77(3):584–592. doi: 10.1161/01.res.77.3.584. [DOI] [PubMed] [Google Scholar]
- Bokesch P. M., Post C., Strichartz G. Structure-activity relationship of lidocaine homologs producing tonic and frequency-dependent impulse blockade in nerve. J Pharmacol Exp Ther. 1986 Jun;237(3):773–781. [PubMed] [Google Scholar]
- Butterworth J. F., 4th, Strichartz G. R. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990 Apr;72(4):711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979 Jul;27(1):39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Chernoff D. M. Kinetic analysis of phasic inhibition of neuronal sodium currents by lidocaine and bupivacaine. Biophys J. 1990 Jul;58(1):53–68. doi: 10.1016/S0006-3495(90)82353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R., Kendig J. J., Cohen E. N. The rates of interaction of local anesthetics with sodium channels in nerve. J Pharmacol Exp Ther. 1978 Nov;207(2):594–604. [PubMed] [Google Scholar]
- Harrison D. C. Antiarrhythmic drug classification: new science and practical applications. Am J Cardiol. 1985 Jul 1;56(1):185–187. doi: 10.1016/0002-9149(85)90591-0. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Bean B. P. Slow binding of phenytoin to inactivated sodium channels in rat hippocampal neurons. Mol Pharmacol. 1994 Oct;46(4):716–725. [PubMed] [Google Scholar]
- McPhee J. C., Ragsdale D. S., Scheuer T., Catterall W. A. A critical role for transmembrane segment IVS6 of the sodium channel alpha subunit in fast inactivation. J Biol Chem. 1995 May 19;270(20):12025–12034. doi: 10.1074/jbc.270.20.12025. [DOI] [PubMed] [Google Scholar]
- Nuss H. B., Tomaselli G. F., Marbán E. Cardiac sodium channels (hH1) are intrinsically more sensitive to block by lidocaine than are skeletal muscle (mu 1) channels. J Gen Physiol. 1995 Dec;106(6):1193–1209. doi: 10.1085/jgp.106.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale D. S., McPhee J. C., Scheuer T., Catterall W. A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994 Sep 16;265(5179):1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A., Porter R. J. Antiepileptic drugs: pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev. 1990 Sep;42(3):223–286. [PubMed] [Google Scholar]
- Sheldon R. S., Hill R. J., Taouis M., Wilson L. M. Aminoalkyl structural requirements for interaction of lidocaine with the class I antiarrhythmic drug receptor on rat cardiac myocytes. Mol Pharmacol. 1991 May;39(5):609–614. [PubMed] [Google Scholar]
- Snyders D. J., Hondeghem L. M. Effects of quinidine on the sodium current of guinea pig ventricular myocytes. Evidence for a drug-associated rested state with altered kinetics. Circ Res. 1990 Feb;66(2):565–579. doi: 10.1161/01.res.66.2.565. [DOI] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Tanguy J. Na channel activation gate modulates slow recovery from use-dependent block by local anesthetics in squid giant axons. Biophys J. 1985 May;47(5):685–694. doi: 10.1016/S0006-3495(85)83965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Dissecting lidocaine action: diethylamide and phenol mimic separate modes of lidocaine block of sodium channels from heart and skeletal muscle. Biophys J. 1993 Dec;65(6):2335–2347. doi: 10.1016/S0006-3495(93)81292-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y. I., Starmer C. F., Grant A. O. Open Na+ channel blockade: multiple rest states revealed by channel interactions with disopyramide and quinidine. Am J Physiol. 1994 May;266(5 Pt 2):H2007–H2017. doi: 10.1152/ajpheart.1994.266.5.H2007. [DOI] [PubMed] [Google Scholar]




