Abstract
Abnormal frontal QRS-T angle on a 12 lead electrocardiogram (ECG) is associated with incident coronary heart disease and total mortality in a biracial cohort but there have been no studies to date examining QRS-T angle’s prognostic value across multiple ethnicities. We studied 6,814 participants (52.7% women, mean age 62) from MESA; a multi-ethnic cohort aged 45–84 free of clinical cardiovascular disease (CVD) at enrollment. Baseline examination included measurement of traditional risk factors and 12-lead ECG’s. Frontal QRS-T axis was defined as normal (<75th percentile), borderline (75–95th percentile) or abnormal (≥ 95th percentile) and participants were followed for the composite endpoint of incident CVD events: cardiovascular death, myocardial infarction, angina pectoris or heart failure. After 7.6 years of follow up there were 444 total events. Borderline ((HR 1.37 95% Confidence Interval (CI) (1.10,1.70)) and abnormal QRS-T angle (HR 2.2 95% CI (1.63, 2.97)) was associated with incident CVD events in multivariable-adjusted models. However, after adjusting for T wave abnormalities there was no statistically significant association of either borderline (HR 1.12 95% CI (0.90, 1.41)) or abnormal (HR 1.31 95% CI (0.93, 1.84)) QRS-T angle with incident CVD events. Abnormal frontal QRS-T angle predicts incident CVD events in a multiethnic population and this increased risk is primarily mediated through T wave abnormalities. QRS-T angle provides an easily interpretable, continuous marker of abnormal ventricular repolarization that can aid the everyday clinician in risk prediction.
Keywords: Electrocardiography, risk assessment, cardiovascular disease
INTRODUCTION
The QRS complex on a surface ECG represents ventricular depolarization and the T wave represents ventricular repolarization. The frontal QRS-T angle, defined as the difference between QRS and T-wave axis, has been described as a marker of ventricular repolarization.1,2 Multiple epidemiologic studies to date have established that abnormal QRS-T angle predicts incident CVD events and mortality.3–9 The vast majority of these studies, however, have been conducted on spatial, as opposed to frontal QRS-T angle. While spatial QRS-T angle is not routinely available in a clinical setting, frontal QRS-T is easily calculated on 12-lead ECG by subtracting T angle from the QRS angle. Moreover, there have been no studies to date examining the association of abnormal QRS-T angle with incident CVD events across a multi-ethnic cohort such as the Multi-Ethnic Study of Atherosclerosis (MESA). Therefore in the present analysis we sought to describe the association of abnormal frontal QRS-T angle with incident CVD events in the MESA.
METHODS
The MESA cohort was initiated to investigate the prevalence, correlates, and progression of subclinical CVD. Details about the study design have previously been published.10 Briefly, between July 2000 and August 2002, 6814 men and women aged 45 to 84 years old, self-identified as white, black, Hispanic, or Chinese and free of clinically apparent CVD were recruited from portions of 6 US communities: Baltimore City and Baltimore County, Maryland; Chicago, Ill; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minn. Each field site recruited from locally available sources, which included lists of residents, lists of dwellings, and telephone exchanges. The institutional review boards at all participating centers approved the study, and all participants gave informed consent.
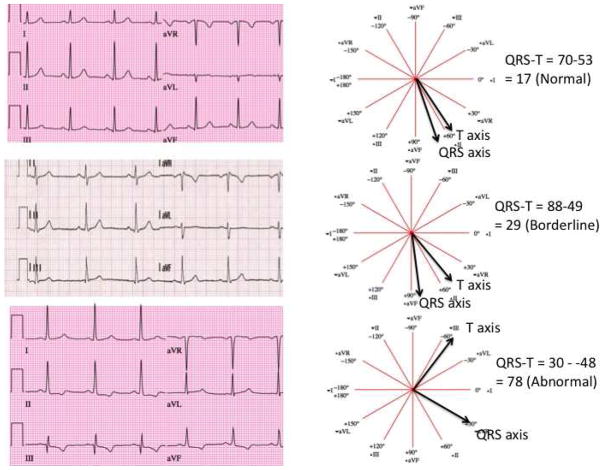
For electrocardiography, three 12-lead recordings were obtained using a Marquette MAC-PC instrument (Marquette Electronics, Milwaukee, Wisconsin). ECG data were processed centrally at the EPICARE center, Wake Forest University using GE Marquette 12-SL 2001 (Milwaukee, Wisconsin) where the ECGs were classified and coded using both Minnesota Code (MC)11 and Novacode criteria (NC)12. For the present analysis baseline ECG’s were utilized and frontal plane QRS-T angle was defined as the absolute value of the difference between the frontal plane QRS axis and T wave axis (if QRS-T angle was > 180 then QRS-T angle = 360 – absolute value). As shown in figure 1 QRS-T angle was defined as normal (<75th percentile), borderline (75th–95th percentile) or abnormal (≥95th percentile). For the purposes of this study, major ECG abnormalities were defined by the presence of 1 or more of the following findings: major ventricular conduction defect, major Q wave, minor Q, QS waves with ST-T abnormalities, major isolated ST-T wave abnormalities, left ventricular hypertrophy, major QT prolongation (≥ 116%), atrial fibrillation or major atrio-ventricular (AV) block. Minor ECG abnormalities were defined by the presence of 1 or more of the following findings: Minor Q, QS waves, high R waves, minor isolated ST-T abnormalities, ST elevation, incomplete (left or right) bundle branch block, minor QT prolongation (≥ 112%), short PR segment (< 120 ms), left axis deviation (>= −90, <= −30), right axis deviation (>=120, <=210), frequent ventricular premature beats, and other minor arrhythmias. ST and T wave abnormalities are defined per Minnesota Code criteria11. Of the 6,814 original participants at baseline, there were 48 participants excluded with missing ECG’s, 87 excluded with incomplete coronary artery calcium (CAC) and carotid intima-medial thickness (C-IMT) measurements, 285 excluded for paced rhythm, Wolf-Parkinson-white pattern, bundle branch block (QRS > =120 msec) or uninterpretable ECG and lastly there were 26 participants excluded for missing follow up data. Hence, there were 6,368 participants available for the current analysis.
Figure 1.
An illustration of the calculation and definition of normal, borderline and abnormal QRS-T angle.
At intervals of 9 to 12 months, an interviewer contacted each participant or a family member by telephone to inquire about interim hospital admissions, outpatient diagnoses of CVD, and deaths. To verify self-reported diagnoses an independent adjudication committee obtained medical records for participants who had been hospitalized or received an outpatient diagnosis of CVD. Interviews were conducted with the next of kin and copies of death certificates were requested for participants who had died of cardiovascular causes outside the hospital. Event definitions have been previously published and are available for reference.10
All analyses were performed using SAS version 9.1 (SAS institute, Cary, NC). Baseline characteristics were described, after stratification by definition of ER, using means and standard deviations (SDs) or percentages. Differences in means or percentages were compared between subgroups using general linear models (t-test) for continuous variables and chi-square tests for categorical variables, as appropriate. Unadjusted and multivariate adjusted Cox proportional hazards regressions models were constructed with QRS-T angle as the predictor (categorized by normal (< 75th percentile), borderline (75–95th percentile) or abnormal (≥95th percentile)) and the composite of cardiovascular death, myocardial infarction, angina or heart failure hospitalization as the outcome. The normal category was considered the reference cell in the analysis and adjustments were made for clinical and ECG variables. In secondary analyses Cox proportional hazards regression models were constructed in sex-specific models adjusting for race as well as race-specific models adjusting for sex. A p value< 0.05 was considered statistically significant.
RESULTS
As shown in Table 1, participants with borderline and abnormal QRS-T angle were older, had higher blood pressure, lower fitness level, and were more likely to be diabetic and have both major and minor ECG abnormalities including ST-T wave abnormalities and ECG-LVH. They were also more likely to have elevated measures of subclinical atherosclerosis (Coronary artery calcium (CAC) > 0, and Common Carotid Intima medial thickness (CC-IMT).
Table 1.
Baseline Characteristics of MESA participants stratified by normal (< 75th percentile), borderline (75–95th percentile) and abnormal (≥95th percentile) QRS-T angle
| Variable | Normal (n=4741) | Borderline (n=1298) | Abnormal (n=329) |
|---|---|---|---|
| Mean QRS-T Angle | 14.9 ± 9.8 | 50.4 ± 11.9 | 104.3 ± 30.1 |
|
| |||
| Age, (years)* | 60.6 ± 9.9 | 65 ± 9.9 | 68.3 ± 9.6 |
|
| |||
| Race | |||
| • White | 38% | 37.6% | 38.3% |
| • Black | 27.7% | 27.1% | 27.1% |
| • Asian | 12.2% | 12.6% | 12.2% |
| • Hispanic | 22.1% | 22.7% | 22.5% |
|
| |||
| Female sex | 53.8% | 53.5% | 54.4% |
|
| |||
| Smoker | 13% | 13.4% | 13.1% |
|
| |||
| Systolic BP, (mm Hg) | 124.5 ± 20.9 | 130.2 ± 21.9 | 138.6 ± 21.9 |
|
| |||
| Diastolic BP, (mm Hg) | 71.6 ± 10.1 | 72.3 ± 10.5 | 74.6 ± 11.3 |
|
| |||
| Body mass index, (kg/m2) | 28.2 ± 5.4 | 28.4 ± 5.6 | 28.9 ± 6.0 |
|
| |||
| Waist:Hip Ratio | 0.92 ± 0.08 | 0.94 ± 0.08 | 0.95 ± 0.07 |
|
| |||
| Total cholesterol, (mg/dL) | 194.6 ± 36.2 | 193.7 ± 34 | 194.9 ± 35.3 |
|
| |||
| HDL-cholesterol, (mg/dL) | 51.3 ± 14.8 | 50.2 ± 14.7 | 51 ± 14.9 |
|
| |||
| LDL-cholesterol, (mg/dL) | 117.5 ± 31.8 | 117.2 ± 30.1 | 116.6 ± 32.8 |
|
| |||
| Triglycerides, (mg/dL) | 130.6 ± 91.9 | 134.3 ± 82.5 | 136.9 ± 78.2 |
|
| |||
| Glucose, (mg/dL) | 96.2 ± 29.9 | 99.8 ± 30.5 | 102 ± 33.6 |
|
| |||
| Creatinine (mg/dl) | 0.94 ± 0.2 | 0.97 ± 0.3 | 1.0 ± 0.3 |
|
| |||
| Diabetes Mellitus | 11.2% | 15.7% | 17.7% |
|
| |||
| Treatment for hypertension | 30% | 38.8% | 46.8% |
|
| |||
| Treatment for hyperlipidemia | 15.2% | 17.8% | 19.5% |
|
| |||
| Physical Activity (MET- min/wk) | 6020 ± 6216 | 5183 ± 4933 | 4604 ± 5210 |
|
| |||
| Common Carotid IMT (mm) | 0.85 ± 0.19 | 0.90 ± 0.21 | 0.94 ± 0.21 |
|
| |||
| QRS (msec) | 91.2 ± 9.5 | 92 ± 10.3 | 93.5 ± 10.5 |
|
| |||
| QTc (msec) | 416.1 ± 20.2 | 420 ± 20.6 | 425.8 ± 24.6 |
|
| |||
| Heart Rate Variability (rmsdd1) (NS) | 26.8 ± 21.7 | 26.4 ± 25.7 | 26.7 ± 31.6 |
|
| |||
| Minor and Major ST abnormalities (Minnesota Code 4-1,4-2,4-3,4-4) | 2.3% | 7.2% | 37.4% |
|
| |||
| Minor and Major T wave abnormalities (Minnesota Code 5-1,5-2,5-3,5-4) | 6.1% | 23.6% | 64.1% |
|
| |||
| Major ECG abnormality (Minnesota Code) | 5.8% | 11% | 44% |
|
| |||
| Minor ECG abnormality (Minnesota Code) | 31.6% | 48.9% | 69.3% |
|
| |||
| ECG-LVH‡ | 0.4% | 0.9% | 10.3% |
|
| |||
| Coronary artery calcium > 0 | 45.9% | 56.6% | 68.4% |
Abbreviations:
BP = Blood Pressure, HDL = High density lipoprotein, LDL = Low density lipoprotein, IMT = intima-medial thickness, LVH = left ventricular hypertrophy
Means ± Standard deviation unless otherwise indicated
Defined as self-reported “yes” or fasting glucose ≥126 mg/dL
Cornell voltage (CV) > 2200μV for women; > 2800μV for men
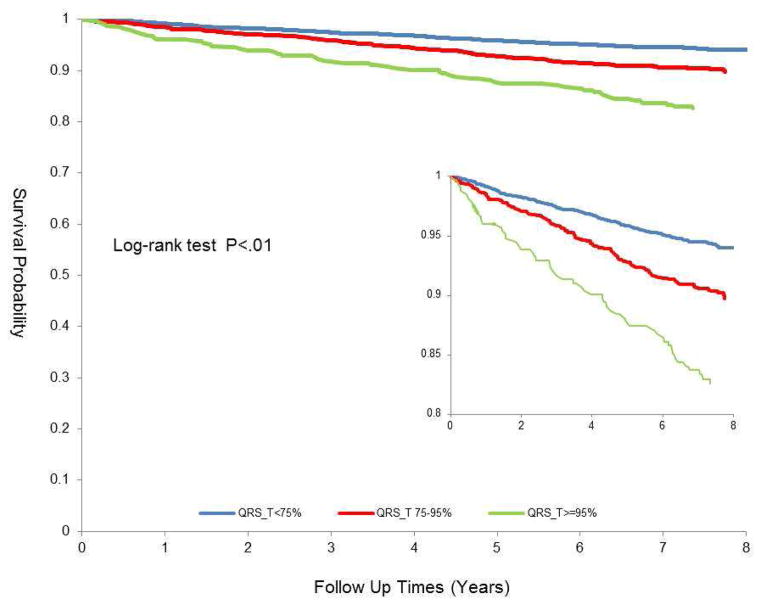
As shown in Table 2 and Figure 2, borderline (75–95th percentile) and abnormal (≥ 95th percentile) QRS-T angle were associated with incident CVD events in models adjusted for age, race and sex ((HR 1.37 95% CI (1.10,1.70)) and (HR 2.2 95% CI (1.63, 2.97), respectively). There was modest attenuation when further adjustment was made for clinical covariates however, after adjustment for major and minor T wave abnormalities there was no statistically significant association of either borderline (HR 1.12 95% CI (0.90, 1.41)) or abnormal (HR 1.31 95% CI (0.93, 1.84)) QRS-T angle.
Table 2.
Unadjusted and multivariable-adjusted Cox proportional hazards ratios for association of QRS-T angle with the outcome of cardiovascular death, myocardial infarction, angina and heart failure
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Normal (< 75th percentile) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Borderline (75th – 95th percentile) | 1.70 (1.37,2.10) | 1.37 (1.10,1.70) | 1.23 (0.99,1.53) | 1.12 (0.90,1.41) |
| Abnormal (≥95th percentile) | 3.12 (2.33,4.17) | 2.20 (1.63,2.97) | 1.75 (1.30,2.37) | 1.31 (0.93,1.84) |
Model 1 – unadjusted
Model 2 – adjusted for age, sex, race
Model 3 – adjusted for age, sex, race, systolic blood pressure, body mass index, triglycerides, QRS duration, QTc duration, smoking status, diabetes, and treatment for hypertension
Model 4 - adjusted for age, sex, race, systolic blood pressure, body mass index, triglycerides, QRS duration, QTc duration, smoking status, diabetes, treatment for hypertension and Minor and Major T wave abnormalities (Minnesota Code 5-1,5-2,5-3 and 5-4)
Figure 2.
Association of normal (< 75th percentile), borderline (75–95th percentile) and abnormal (>95th percentile) QRS-T angle with CVD outcomes of cardiovascular death, myocardial infarction, angina and heart failure (Kaplan Meier)
In secondary analyses, the association of abnormal QRS-T angle with incident CVD events remained in both sex-specific models adjusting for race as well as race-specific models adjusting for sex with the exception of Chinese men and women. However, the low event rate in this subgroup suggests that the model was underpowered to show an association.
DISCUSSION
Our findings show that in a multiethnic population of adults aged 45–84 years free of CVD at enrollment abnormal frontal QRS-T angle was associated with incident CVD events in multivariable-adjusted models. However, after further adjustment for major and minor T wave abnormalities, the association with CVD was attenuated to non-significance. Our study validates the role of frontal QRS-T angle in risk prediction of CVD among a multi-ethnic cohort and suggests that it is primarily mediated through T wave abnormalities. However, as opposed to dichotomous cut points for repolarization abnormalities such as abnormal T wave or ST abnormities, QRS-T angle provides an easily interpretable, continuous marker of abnormal ventricular repolarization that can aid the everyday clinician in risk prediction.
Numerous epidemiologic studies to date have established that abnormalities in repolarization as manifested by ST deviation and T wave abnormalities are strong risk markers for incident CVD events. These abnormalities likely reflect electrophysiological manifestations of structural heart disease and electrical perturbations from ion channel abnormalities that mediate this increased risk for CVD. However, as opposed to binary coding variables such as non-specific ST and T wave abnormalities or major / minor T wave abnormalities, QRS-T angle is a continuous variable that can be used to easily assess abnormal ventricular repolarization across a range of abnormal values from borderline to significantly abnormal. Hence, QRS-T angle may play a role in quantifying the degree of abnormal repolarization and detect repolarization abnormalities before overt ECG changes (i.e. T wave inversion or ST depression) occur.
Initial investigation into QRS-T angle by Kardys4 and Yamazaki5 demonstrated that spatial QRS-T angle was associated with incident CVD and total mortality in a graded fashion. Spatial QRS-T angle, however, is not routinely calculated on the 12 lead ECG and less available for the every day clinician. Subsequent analysis by Zhang9 from the ~ 14,000 participants in the ARIC study demonstrated that frontal QRS-T angle, defined as the T wave axis minus QRS axis on the standard 12 lead ECG, demonstrates similar predictive utility to spatial QRS-T for total mortality. Zhang and colleagues went on to show that QRS-T angle was predictive of incident CHD only in women, with spatial QRS-T showing improved risk prediction for CHD events compared to frontal QRS-T. Similar to our analysis, when ST-T abnormalities were added to a risk prediction model using clinical variables, the utility of QRS-T angle for prediction both total mortality and incident CHD was significantly attenuated. Our findings validate the ARIC analysis in a large multi-ethnic cohort (ARIC was only biracial) and further suggest that QRS-T is a strong predictor of incident CVD events across ethnicity that is primarily mediated by T wave abnormalities. Hence, QRS-T angle is an easily interpretable and continuous electrocardiographic marker of CVD risk that can play a critical role in risk prediction, particularly among patients without overt ECG abnormalities.
The lack of spatial QRS-T angle in our analysis may limit our findings as the utility of frontal QRS-T angle has only been validated in the ARIC study as mentioned above. However, the utility of spatial, as opposed to frontal QRS-T angle, was only superior in the prediction of incident CHD among women from the ARIC cohort. In addition, while QRS-T angle’s ability to predict incident CVD was attenuated to non-significance in models with T wave abnormalities, the confidence intervals were wide and this analysis was likely underpowered to demonstrate the albeit small residual utility of QRS-T angle beyond T wave abnormalities.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
CONFLICTS OF INTEREST: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dilaveris P, Gialafos E, Pantazis A, Synetos A, Triposkiadis F, Gialafos J. The spatial QRS-T angle as a marker of ventricular repolarisation in hypertension. J Hum Hypertens. 2001;15:63–70. doi: 10.1038/sj.jhh.1001129. [DOI] [PubMed] [Google Scholar]
- 2.Dilaveris P, Gialafos E, Pantazis A, Synetos A, Triposkiadis F, Stamatelopoulos S, Gialafos J. Spatial aspects of ventricular repolarization in postinfarction patients. Pacing Clin Electrophysiol. 2001;24:157–165. doi: 10.1046/j.1460-9592.2001.00157.x. [DOI] [PubMed] [Google Scholar]
- 3.Lipton JA, Nelwan SP, van Domburg RT, Kors JA, Elhendy A, Schinkel AF, Poldermans D. Abnormal spatial QRS-T angle predicts mortality in patients undergoing dobutamine stress echocardiography for suspected coronary artery disease. Coron Artery Dis. 2010;21:26–32. doi: 10.1097/MCA.0b013e328332ee32. [DOI] [PubMed] [Google Scholar]
- 4.Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–64. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women’s Health Initiative. Circulation. 2006;113:473–480. doi: 10.1161/CIRCULATIONAHA.104.496091. [DOI] [PubMed] [Google Scholar]
- 7.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in postmenopausal women: the Women’s Health Initiative. Circulation. 2006;113:481–489. doi: 10.1161/CIRCULATIONAHA.105.537415. [DOI] [PubMed] [Google Scholar]
- 8.Rautaharju PM, Prineas RJ, Wood J, Zhang ZM, Crow R, Heiss G. Electrocardiographic predictors of new-onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study) Am J Cardiol. 2007;100:1437–1441. doi: 10.1016/j.amjcard.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM. Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study) Am J Cardiol. 2007;100:844–849. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Prineas RJCR, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA: John Wright - PSG, Inc; 1982. p. 330. [Google Scholar]
- 12.Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31:157–187. [PubMed] [Google Scholar]