Abstract
Purpose
The aim of this study is to assess results of treatment, factors influencing prognosis with regard to causes of failure and treatment tolerance in patients with thymoma.
Material and methods
Between 1966 and 2006, 63 patients with thymoma had been treated at the Centre of Oncology in Krakow. Patients were treated by means of different treatment modalities: surgery followed by radiotherapy (52%), radiotherapy alone (13%), chemoradiotherapy alone (15%), surgery followed by chemoradiotherapy (5%), surgery alone (5%) and others.
Results
The 10-year locoregional recurrence-free survival (LRRFS) was 79%, disease free survival (DFS) was 57% and overall survival (OS) was 57%. Masaoka stage was the only independent prognostic factor for LRRFS. Masaoka stage and method of radiotherapy delivery (higher photon energies), were independent prognostic factors for OS. For DFS, the independent prognostic factors were age, type of treatment (favoured surgery followed by radiotherapy or chemoradiotherapy), Masaoka stage and year of start of treatment. Most common reactions were lung fibrosis in 36% of patients (mainly asymptomatic in most patients), pneumonitis (9%) and oesophagitis (4%).
Conclusions
Surgery combined with radiotherapy and chemoradiotherapy and modern radiotherapy techniques are correlated with improvement of survival in patients with early stage thymoma.
Keywords: Thymoma, Radiotherapy, Surgery, Prognostic factors
1. Introduction
Thymomas, which originate from epithelial cells of the thymus, are the most common tumours of the anterosuperior mediastinum and represent about 20–30% of all malignant mediastinal tumours.1,2 These tumours are rare neoplasms; the age-standardized incidence rates range from 0.1 to 0.5 per 100 000 population annually in different countries.3 Data regarding incidence of thymomas in Poland are not available.
Usually thymomas are asymptomatic (the tumour is detected by incidental chest X-rays) or associated with symptoms such as myasthenia gravis. The Masaoka classification has been the most widely used staging system.4,5
Due to the low incidence of these tumours (which precludes large randomized trials), little is known about optimal evidence-based therapy of thymomas.
Surgery still remains the principal method of treatment of resectable thymomas, whereas adjuvant or neoadjuvant radiotherapy plays an important role in subtotally resected and unresectable cases.1,6,7 In advanced cases (Masaoka stages III and IV) or in patients with recurrence after the treatment, chemotherapy can prolong survival and offers palliation. Advanced thymomas should be treated by a multidisciplinary team including surgeons, and medical and radiation oncologists.8
The aim of this study is to assess the results of treatment in patients with thymoma, factors influencing prognosis with regard to causes of failure and treatment tolerance.
2. Materials and methods
Retrospective analysis was performed in a group of 63 patients with thymoma, who had been treated at the Centre of Oncology in Krakow between 1966 and 2006. Characteristics of patients are shown in Table 1. Median age was 43 years, 31 patients were males, 32 were females. About 70% of patients were in stages III and IV. The majority (71%) of patients presented symptoms preceding diagnosis of thymomas; the most common were chest pain with dyspnoea, flu-like symptoms and myasthenia gravis. In all cases diagnosis was confirmed by histology.
Table 1.
Characteristics of patients.
| Characteristics | n | % |
|---|---|---|
| Age | ||
| Median | 43 | |
| Range | 17–74 | |
| Gender | ||
| Female | 32 | 51% |
| Male | 31 | 49% |
| Symptoms before treatment | ||
| Absent | 18 | 29% |
| Present | 45 | 71% |
| Stage (Masaoka) | ||
| I | 8 | 13% |
| IIa | 4 | 6% |
| IIb | 6 | 10% |
| IIIa | 18 | 29% |
| IIIb | 19 | 30% |
| IVa | 4 | 6% |
| IVb | 2 | 3% |
| Not applicable | 2 | 3% |
| Type of treatment | ||
| Surgery followed by radiotherapy | 33 | 52% |
| Radiotherapy alone | 8 | 13% |
| Chemotherapy | 2 | 3% |
| Chemoradiotherapy | 9 | 14% |
| Palliative care | 1 | 2% |
| Surgery | 3 | 5% |
| Surgery followed by chemoradiotherapy | 5 | 8% |
| Surgery followed by chemotherapy | 2 | 3% |
| Completeness of operation | ||
| R0 | 16 | 37% |
| R1 | 4 | 9% |
| R2 | 14 | 33% |
| Not applicable | 9 | 29% |
Patients were treated using different treatment modalities: surgery followed by radiotherapy (52%), radiotherapy alone (13%), chemoradiotherapy alone (15%), surgery followed by chemoradiotherapy (5%), surgery alone (5%) and others.
Median radiation dose was 4600 cGy (range 3000–7000) in 23 fractions (median). Radiotherapy was delivered by linear accelerator (37 pts), cobalt (18 pts) and ortovoltage (1 pts) because of development of methods of radiotherapy delivery technologies during the 40 years of this retrospective analysis.
Indications for irradiation in our hospital were: subtotally resected, all Masaoka stages II and III in adjuvant setting, and unresectable thymoma.
Techniques of radiation delivery have changed during the 40 years of this analysis. Irradiated volume included whole thymus, tumour bed, mediastinum. Two antero-posterior field techniques were used in the first phase of treatment. Dose to the spinal cord was limited to 45 Gy using two or three oblique fields in the second phase. The majority (60%) of patients treated with chemotherapy received anthracycline- and cisplatin-based regimens. All of them were treated in the adjuvant setting.
In the radiochemotherapy subgroup patients subsequently received nitrogranulogen (3 patients) or anthracycline-based chemotherapy (6 patients).
2.1. Statistical methods
The main endpoints of the analysis were locoregional recurrence-free survival (LRRFS), disease-free survival (DFS) and overall survival (OS) rates.
Survival was measured from the date of diagnosis to the date of death or last follow-up.
Locoregional recurrence-free survival was measured from the date of treatment to the date of locoregional recurrence or death or last follow-up in the subgroup of patients after complete resection (confirmed in imaging studies) and patients treated with other modalities with complete response.
Time to progression was measured from the date of the end of treatment to the date of local or distant progression. Local failure was defined as failure occurring inside the mediastinum (and/or with invasive growth into neighbouring organs) and distant failure as any site of failure outside the mediastinum.
Kaplan–Meier method was used to calculate survival rates. Univariate and multivariate analyses of prognostic factors were performed using log rank and Cox's proportional hazard method. In analyses of prognostic factors, clinical factors (sex, age, stage, grade, histological type, symptoms, etc.) and treatment factors (method of resection, completeness, radiation dose, total treatment time, year of start of treatment) were compared. P-Values of less than 0.05 were considered to indicate statistical significance.
3. Results
3.1. Survival
During a median follow-up of 90 months (range 3–361), we observed 22 cancer-related deaths and 11 patients died from other causes. About half of patients (30) were alive in May 2007, of whom 24 were disease free.
The 10-year LRRFS rate, DFS rate and OS (Fig. 1) rate were 79, 57 and 57%, respectively.
Fig. 1.
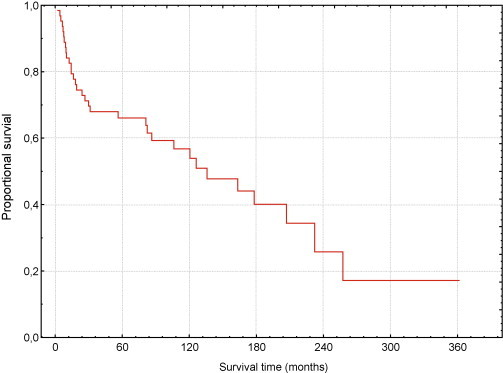
Overall survival.
The univariate analysis (Table 2) showed that completeness of operation and Masaoka stage had a significant impact on LRRFS. LRRFS in the subgroup of patients after complete resection (confirmed in imaging studies) and patients treated with other modalities with complete response were 86 and 66%, respectively (P = 0.2).
Table 2.
Univariate analysis of prognostic factors (significant bolded).
| Prognostic factors | Number of pts. | % | 10-Year LRFS% | 10-Year DFS% | 10-Year OS% |
|---|---|---|---|---|---|
| Total group | 63 | 100% | 79% | 57% | 57% |
| Gender | |||||
| Female | 32 | 51% | 78% | 56% | 56% |
| Male | 31 | 49% | 79% | 57% | 58% |
| Age | P = 0.007 | ||||
| >43 years | 33 | 52% | 79% | 72% | 67% |
| ≤43 years | 30 | 48% | 78% | 41% | 47% |
| Histological subtype (Bernatz classification) | |||||
| Not applicable | 33 | 53% | 71% | 43% | 44% |
| Epithelialthymoma | 8 | 13% | 100% | 66% | 50% |
| Lymphocytic thymoma | 4 | 6% | 100% | 75% | 75% |
| Mixed thymoma | 11 | 17% | 87.5% | 67.5% | 80% |
| Spindlecell thymoma | 2 | 3% | 100% | 100% | 100% |
| Thymic carcinoma | 5 | 8% | 0% | 50% | 40% |
| Symptoms before treatment | |||||
| Absent | 18 | 29% | 85% | 74% | 81% |
| Present | 45 | 71% | 74% | 49% | 47% |
| Symptoms (described) | |||||
| Myasthenia gravis | 9 | 14% | 50% | 50% | 52% |
| Chest pain, dyspnoea | 23 | 36% | 77% | 53% | 52% |
| Cough | 2 | 3% | 100% | 100% | 50% |
| Hoarseness | 1 | 1.5% | NA | 0% | 0% |
| Dysphagia | 1 | 1.5% | NA | NA | NA |
| Fatigue | 1 | 1.5% | NA | NA | NA |
| Flu-like symptoms | 8 | 12.5% | NA | 22% | 42% |
| Type of treatment | P = 0.03 | P = 0.002 | |||
| Surgery followed by radiotherapy | 33 | 52% | 87% | 81% | 77% |
| Radiotherapy alone | 8 | 13% | 66% | 39% | 25% |
| Chemotherapy | 2 | 3% | NA | 0% | 0% |
| Chemoradiotherapy | 10 | 15% | 0% | 0% | 0% |
| Surgery | 3 | 5% | 100% | 100% | 100% |
| Surgery followed by chemoradiotherapy | 5 | 8% | 0% | 0% | 80% |
| Surgery followed by chemotherapy | 2 | 3% | 100% | 0% | 50% |
| Completeness of operation | P = 0.04 | P = 0.03 | P = 0.001 | ||
| R0 | 16 | 37% | 100% | 91% | 100% |
| R1 | 4 | 9% | 100% | NA | 75% |
| R2 | 14 | 33% | 73% | 47% | 71% |
| No data | 9 | 29% | 59% | 35% | 27% |
| Masaoka stage | P = 0.04 | P = 0.02 | P = 0.004 | ||
| I | 8 | 13% | 100% | 83% | 100% |
| IIa | 4 | 6% | NA | NA | 100% |
| IIb | 6 | 10% | NA | NA | 100% |
| IIIa | 18 | 29% | 83% | 65% | 71% |
| IIIb | 19 | 30% | 42% | 23% | 28% |
| IVa | 4 | 6% | 0% | 0% | 0% |
| IVb | 2 | 3% | NA | NA | 0% |
| No data | 2 | 3% | NA | 0% | 0% |
| Radiotherapy | P = 0.04 | P = 0.001 | |||
| Without radiotherapy | 7 | 11.1% | NA | 33% | 33% |
| Ortovoltage | 1 | 1.6% | NA | 0% | 0% |
| Cobalt | 18 | 28.6% | 80% | 58% | 35% |
| Linear accelerator | 37 | 58.7% | 73% | 57% | 73% |
| Year of start of treatment | P = 0.01 | P = 0.005 | |||
| 1966–1991 | 31 | 49% | 81% | 50% | 42% |
| 1992–2006 | 32 | 51% | 71% | 62% | 74% |
| Radiation dose | |||||
| Without radiotherapy | 7 | 11.1% | |||
| <4600 | 27 | 42.9% | 84% | 58% | 53% |
| ≥4600 | 29 | 46% | 63% | 52% | 59% |
NA, not applicable.
The following prognostic factors significantly influenced DFS: age, type of treatment, completeness of operation, Masaoka stage, method of radiotherapy delivery and year of start of treatment.
Type of treatment, completeness of operation, Masaoka stage, year of start of treatment and method of radiotherapy delivery were statistically significant prognostic factors for OS.
Masaoka stage was the only independent prognostic factor for LRRFS (Table 3). The risk of locoregional recurrence in patients with early Masaoka stages was less than half of the risk in advanced stage patients.
Table 3.
10 Years multivariate analysis of prognostic factors.
| Variable | Relative risk | P-Value | |
|---|---|---|---|
| LRFS | Masaoka stage | 1.8 | 0.004 |
| DFS | Age | 0.91 | 0.00069 |
| Type of treatment | 1.78 | 0.000199 | |
| Masaoka stage | 1.56 | 0.0088 | |
| Year of start of treatment | 0.16 | 0.04 | |
| OS | Masaoka stage | 2.10 | 0.0000 |
| Radiotherapy | 0.43 | 0.0006 | |
In the multivariate analysis, the following prognostic factors had a statistically significant impact on recurrence rate: age (favoured older patients), type of treatment, Masaoka stage (favoured early stage patients) and year of start of treatment (favoured patients treated after 1991) (Table 3).
For OS (Table 3), the independent prognostic factors were Masaoka stage and method of radiotherapy delivery (higher photon energies). The risk of death was more than two times higher in advanced Masaoka stage patients and in those who were treated with older methods of radiotherapy delivery. Ten-year OS was 100, 100, 71, 28 and 0% in stages I, II, IIIA, IIIB and IV, respectively.
Neither symptoms (myasthenia gravis, chest pain, dyspnoea) nor radiotherapy dose, irradiated volume, or histological stage appeared to be of prognostic significance in this group of patients.
In 25 patients treated with chemoradiotherapy, radiotherapy, chemotherapy or surgery (in the case of macroscopically non-radical operation) follow-up imaging studies revealed complete response in 9 patients, partial response in 2, stable disease in 6 and progressive disease in 8.
3.2. Analysis of failure
During the follow-up, local recurrence of malignancy was observed in 8 patients; half of them were located outside the irradiated volume. In 16 patients distant failure was observed. In 8 cases (58%) distant failure was located in the lungs, in 3 (36%) it was found in the liver, and in 2 in the brain. Median time to distant recurrence was 18 months (6–76).
3.3. Safety
Radiation therapy was well tolerated in 55% of patients (31 of 56 radiotherapy patients). The most common reactions were lung fibrosis in 36% of patients (asymptomatic in the majority of patients), pneumonitis (9%) and oesophagitis (4%).
There were two serious (grade 3) side effects: lung fibrosis and oesophagitis. Severities of the reactions are shown in Table 4. Incidence of reactions was correlated with age and total dose. Patients who complained of reactions were older (48 years vs 40 years) and were irradiated with a higher dose (median 4868 cGy vs 4283 cGy) compared to asymptomatic patients.
Table 4.
Treatment complications (number of patients).
| Complication | EORTC/RTOG scale |
||
|---|---|---|---|
| I | II | III/IV | |
| Lung fibrosis | 11 | 8 | 1 |
| Pneumonitis | 2 | 2 | 1 |
| Oesophagitis | 0 | 2 | 0 |
Four patients (6%) had a secondary malignancy (NHL, malignant melanoma, breast cancer, non-small lung cancer). All secondary tumours were diagnosed later than thymoma (7–190 months). The patients with second malignancy presented more advanced thymoma than patients without secondaries (P = 0.037). No statistically significant differences were demonstrated in other variables: age, gender, type of treatment, radiotherapy dose, radiotherapy volume, histological type and symptoms.
4. Discussion
The low incidence of thymomas precludes conducting prospective trials and evidence is based on small retrospective studies with moderate numbers of included patients. We found only one randomized control trial of thymoma treatment.9 There are also no thymoma treatment practice guidelines.
The retrospective design is a limitation of the study. Over the 40 years of observation there have been many changes and improvements of both diagnostics and therapy. Due to the low patient number it is difficult to perform adequate subgroup analysis.
Clinical characteristics of our series of patients are similar to those reported in the literature. Sex distribution was balanced and most patients were in the fifth decade of life.3,7 Incidence of symptoms most commonly reported by the literature – chest pain, dyspnoea (36%) and myasthenia gravis (14%) – were lower than reported in other series, which may be influenced by interviewer bias, typical for retrospective methodology.2,10,11,12
Overall survival rates for stages I, II and IIIa are higher whereas for stages IIIB and IV are lower than reported elsewhere.10,11,13 Survival for the whole group of patients (57%) is lower than in other series due to the fact that about 70% were advanced (Masaoka stages III and IV), while in the literature 50% are diagnosed when they are localised within the capsule and do not infiltrate.7
With respect to prognosis in our series Masaoka stage was a prognostic factor of LRRFS, DFS and OS.11,13–17 Patients who started treatment after 1991 had fewer recurrences than those who were treated earlier. This could be explained by the fact of improvement of imaging and radiation delivery methods and the common introduction of anthracyclines into adjuvant treatment. Patients older than 43 had longer DFS, which could be attributed to aggressiveness (histological grade). Nevertheless, this observation is opposite to the majority of results published in other series (with the exception of Resbeut's study).2,11,13,18
In contrast, patient sex, histological subtype and presence of myasthenia gravis and radiotherapy dose show no correlation with survival, which is consistent with the findings of a number of published studies.2,13
During 40 years five different classifications were proposed by Bernatz, Rosai and Levine, Müller and Hermelink, Suster and Moran, and the WHO, which still seem to be controversial.19,20 In our group of patients the Bernatz classification was used most often (47%). Nevertheless, a comparison could not be performed, which is a limitation of our study.
Surgical margin status was not an independent prognostic factor in multivariate analysis (only in univariate), unlike in other series.17,21
In our series distant relapses are two times more common than local ones, which can be explained by low sensitivity and specificity of detection of local recurrence (frequently asymptomatic and later can lead to distant failure) and higher number of patients with advanced disease compared to other series.2
In 25 patients treated with chemoradiotherapy, radiotherapy, chemotherapy or surgery (in the case of macroscopically non-radical operation) response rate assessed by follow-up imaging studies was 44%, whereas in other studies it is heterogeneous and ranges between 24 and 100%.22,23
Radiotherapy was well tolerated in the majority of patients. Most reactions were asymptomatic. The frequency and severity of complications of both chemotherapy and radiotherapy was low; nevertheless, this result should be interpreted with caution because of underreporting of side effects in retrospective studies.
An increased risk of developing secondary malignancy has been suspected in thymoma, with a reported incidence from 3 to 22%.24 Reported incidence of secondary malignancies is 6%, which correlates with Masaoka stage only. No other studied factor influenced incidence of secondaries.
5. Conclusions
Surgery combined with radiotherapy and chemoradiotherapy and modern radiotherapy techniques (three-dimensional treatment planning, CT imaging, higher photon energies) are correlated with improvement of survival in patients with early stage thymoma. In patients with very early stage thymoma, complete surgical excision seems to be a sufficient method of treatment.
References
- 1.Graeber G.M., Tamim W. Current status of the diagnosis and treatment of thymoma. Semin Thorac Cardiovasc Surg. 2000;12:268–277. doi: 10.1053/stcs.2000.17955. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa K., Uno T., Toita T., Onishi H., Yoshida H., Kakinohana Y. Postoperative radiotherapy for patients with completely resected thymoma: a multi-institutional, retrospective review of 103 patients. Cancer. 2002;94:1405–1413. doi: 10.1002/cncr.10373. [DOI] [PubMed] [Google Scholar]
- 3.Engels E.A., Pfeiffer R.M. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–551. doi: 10.1002/ijc.11099. [DOI] [PubMed] [Google Scholar]
- 4.Masaoka A., Monden Y., Nakahara K., Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Asamura H., Nakagawa K., Matsuno Y., Suzuki K., Watanabe S., Tsuchiya R. Thymoma needs a new staging system. Interact Cardiovasc Thorac Surg. 2004;3(1):163–167. doi: 10.1016/S1569-9293(03)00265-2. [DOI] [PubMed] [Google Scholar]
- 6.Eng T.Y., Thomas C.R., Jr Radiation therapy in the management of thymic tumors. Semin Thorac Cardiovasc Surg. 2005;17:32–40. doi: 10.1053/j.semtcvs.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Giaccone G. Treatment of malignant thymoma. Curr Opin Oncol. 2005;17:140–146. doi: 10.1097/01.cco.0000152628.43867.8e. [DOI] [PubMed] [Google Scholar]
- 8.Evans T.L., Lynch T.J. Role of chemotherapy in the management of advanced thymic tumors. Semin Thorac Cardiovasc Surg. 2005;17:41–50. doi: 10.1053/j.semtcvs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H., Lu N., Wang M., Gu X., Zhang D. Postoperative radiotherapy for stage I thymoma: a prospective randomised trial in 29 cases. Chin Med J (Engl) 1999;112:136–138. [PubMed] [Google Scholar]
- 10.Kondo K., Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878–884. doi: 10.1016/s0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 11.Gawrychowski J., Rokicki M., Gabriel A., Lackowska B., Czyzewski D. Thymoma—the usefulness of some prognostic factors for diagnosis and surgical treatment. Eur J Surg Oncol. 2000;26:203–208. doi: 10.1053/ejso.1999.0777. [DOI] [PubMed] [Google Scholar]
- 12.Sunpaweravong P., Kelly K. Treatment of thymoma: a comparative study between Thailand and the United States and a review of the literature. Am J Clin Oncol. 2004;27:236–246. doi: 10.1097/01.coc.0000092561.21421.88. [DOI] [PubMed] [Google Scholar]
- 13.Zhu G., He S., Fu X., Jiang G., Liu T. Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys. 2004;60:1113–1119. doi: 10.1016/j.ijrobp.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Johnson S.B., Eng T.Y., Giaccone G., Thomas C.R., Jr Thymoma: update for the new millennium. Oncologist. 2001;6:239–246. doi: 10.1634/theoncologist.6-3-239. [DOI] [PubMed] [Google Scholar]
- 15.D’Angelillo R.M., Trodella L., Ramella S., Cellini N., Balducci M., Mantini G. Novel prognostic groups in thymic epithelial tumors: assessment of risk and therapeutic strategy selection. Int J Radiat Oncol Biol Phys. 2008;71(2):420–427. doi: 10.1016/j.ijrobp.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Kim B.K., Cho B.C., Choi H.J., Sohn J.H., Park M.S., Chang J. A single institutional experience of surgically resected thymic epithelial tumors over 10 years: clinical outcomes and clinicopathologic features. Oncol Rep. 2008;19(6):1525–1531. [PubMed] [Google Scholar]
- 17.Sousa B., Araújo A., Amaro T., Azevedo I., Soares M., Sousa O. Malignant thymomas—the experience of the Portuguese Oncological Institute, Porto, and literature review. Rev Port Pneumol. 2007;13(4):553–585. [PubMed] [Google Scholar]
- 18.Resbeut M., Mornex F., Richaud P. Radiotherapy of thymoma. Study of the literature apropos of a retrospective and multicenter series of 149 cases. Bull Cancer Radiother. 1995;82:9–19. [PubMed] [Google Scholar]
- 19.Park M.S., Chung K.Y., Kim K.D. Prognosis of thymic epithelial tumors according to the new World Health Organization histologic classification. Ann Thorac Surg. 2004;78:992–997. doi: 10.1016/j.athoracsur.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 20.Szołkowska M., Langfort R. Thymomas—review and usefulness of applied classifications. Onkol Pol. 2000;3:149–154. [Google Scholar]
- 21.Myojin M., Choi N.C., Wright C.D., Wain J.C., Harris N., Hug E.B. Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. Int J Radiat Oncol Biol Phys. 2000;46:927–933. doi: 10.1016/s0360-3016(99)00514-3. [DOI] [PubMed] [Google Scholar]
- 22.Hejna M., Haberl I., Raderer M. Nonsurgical management of malignant thymoma. Cancer. 1999;85:1871–1884. [PubMed] [Google Scholar]
- 23.Jacot W., Quantin X., Valette S., Khial F., Pujol J.L. Multimodality treatment program in invasive thymic epithelial tumor. Am J Clin Oncol. 2005;28(1):5–7. doi: 10.1097/01.coc.0000138963.01562.d3. [DOI] [PubMed] [Google Scholar]
- 24.Pan C.C., Chen P.C., Wang L.S., Chi K.H., Chiang H. Thymoma is associated with an increased risk of second malignancy. Cancer. 2001;92:2406–2411. doi: 10.1002/1097-0142(20011101)92:9<2406::aid-cncr1589>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


