Abstract
Background
Radical cystectomy is the standard treatment for patients with muscle-invasive urinary bladder cancer; however, is associated with major treatment – related morbidity. Furthermore, a significant proportion of patients are deemed unsuitable for surgery due to inoperability, advanced age, and/or comorbid conditions. As such, several groups have explored effectiveness of less radical therapeutic strategies that aim at bladder preservation. Nonetheless, there is scarcity of reports assessing the applicability of urinary bladder-sparing outside developed countries.
Aim
Determine the achievable outcomes for patients with muscle-invasive urinary bladder cancer treated via bladder-sparing techniques in a low income country.
Materials and methods
Fourteen consecutive patients with a diagnosis of muscle-invasive urinary bladder cancer (clinical stage; T2-3N0M0) were treated via a bladder-sparing approach at King Hussein Cancer Center (Amman, Jordan) between 2005 and 2009. Records were electronically retrieved and retrospectively analyzed and included 11 males and 3 females from 41 to 74 years of age (median age, 61). Initial therapy consisted of trans-urethral resection of bladder tumor (TURBT) followed by induction chemotherapy then irradiation (4500cGy) with concurrent platinum-based chemotherapy. Urological evaluation directed additional therapy in a proportion of patients with irradiation (up to 6400 cGy) in patients who achieved CR.
Results
Eleven patients were evaluable for pathological response at time of re-staging; of whom 8 (73%) achieved CR and 3 (27%) achieved partial response (PR). In all but one patient; combined-modality treatment was well tolerated. After a median follow-up of 18.5 months (range, 3–48 months); 5 of 8 (62.5%) patients with CR were alive.
Conclusions
Bladder-sparing strategies via concurrent chemoradiation for muscle-invasive bladder cancer results in an acceptable rate of complete pathological response with adequate short-term outcomes. This approach appears applicable in low-income countries.
Keywords: Urinary bladder, Cancer, TURBT, Low-income, Bladder-preservation
Abbreviations: CR, complete response; PR, partial response; TURBT, transurethral resection of bladder tumor; TCCB, transitional cell carcinoma of the bladder; cCRT, concurrent chemoradiation
1. Background
Urinary bladder cancer continues to pose a significant global health challenge. Transitional cell carcinoma is the most prevalent pathological subtype and approximately 30% of such cases are muscle-invasive at the time of diagnosis [100,2]. Radical cystectomy is the standard therapy for these patients with an expected 5-year survival of 45–60%. However, radical surgery comes at the cost of long-term morbidity secondary to urinary diversion techniques.3 As a consequence, numerous studies have explored the application of non-surgical strategies in the management of muscle-invasive bladder cancer.4 Nonetheless, there is scarcity of reports assessing bladder preservation outside developed countries. We present our experience with the use of combined chemoradiation in such patients in a low-income setting.
2. Aim
The aim of this report is to assess the applicability and determine the achievable outcomes for patients with muscle-invasive urinary bladder cancer treated via bladder-sparing strategies in a low income country. We intend to report the indications, complications and therapeutic outcomes of chemoradiation in these patients. This includes special reference to the rate of complete pathological response obtained after combined-modality therapy. In addition, we will outline the utilized radiotherapy planning technique. Furthermore, we intend to shed light on the current status of oncological healthcare in Jordan focusing on the challenges of successfully implementing bladder-preservation strategies for muscle-invasive urinary bladder cancer in low-income countries.
3. Patients and methods
Between March 2005 and June 2008, fourteen patients with biopsy-proven muscle invasive transitional cell carcinoma of the bladder (TCCB) were treated via a bladder-sparing approach at the King Hussein Cancer Center (Amman, Jordan). Records were electronically retrieved and retrospectively analyzed following the acquisition of Institutional Review Board approval and included 11 males and 3 females from 41 to 74 years of age (median age 61). Exhaustive chart analysis was performed in an attempt to extract data pertaining to pathological characters, clinical stage, treatment, disease outcome and survival. Patients were evaluated through history, complete physical examination and appropriate radiological (CT chest, abdomen and pelvis) and nuclear imaging studies (bone scan). Pathological diagnosis was confirmed by examining/re-examining biopsy specimens obtained via trans-urethral biopsy by a staff pathologist at the KHCC. All tumor specimens demonstrated malignant invasion of muscularis propia. Patients were clinically classified according to the 2002 American Joint Committee on Cancer-International Union Against Cancer classification.5 Patients were excluded if there was evidence of distant metastases or if chemoradiation was inappropriate or contraindicated. Cases were discussed at a joint care conference prior to therapeutic decisions and delivery of care. Radical cystectomy was inapplicable due to medical contraindications, patient unsuitability or refusal. Initial therapy consisted of TURBT (as aggressive as judged to be safely possible) followed by induction chemotherapy, then irradiation with concurrent platinum-based agents. Complete blood counts and biochemical studies were obtained prior to each dose of chemotherapy. The dose of cisplatin and gemcitabine was set at 70 mg/m2 and 1000 mg/m,2 respectively. Carboplatin was administered instead of cisplatin to patients with mild renal impairment as bolus infusion once a week, up to a total dose of 900 mg. The dose of cisplatin was reduced if the serum creatinine concentration rose above 1.5 mg/dL. Severe hematological toxicity was managed via a temporary discontinuation of chemotherapeutic agents. Radiation therapy was delivered via an Elekta linear accelerator (Elekta Oncology Systems, Crawley, UK). The bladder and pelvic lymphatics were treated via a four-field box technique to a total dose of 4500 cGy given over a period of 5 weeks (180cGy daily fractions in 5 consecutive days). Planned target volume (PTV) consisted of the bladder and tumor with 2-cm contoured margin (Fig. 1). Re-staging cystectomy performed 4 weeks after the completion of cCRT directed additional therapy with delivery of irradiation (up to 6400 cGy) in a proportion of patients who had CR (Fig. 2). A complete pathological response required demonstration of absence of any macroscopically and microscopically viable tumor in addition to negative urine cytology. Informed written consent was obtained from all patients. Follow-up was in the form of a patient visit to a staff clinician at the KHCC, a patient follow-up phone interview or retrieval from the institutional-tumor registry and national data archives. The length of the follow-up ranged from 3 to 48 months, with a median follow-up period of 18.5 months.
Fig. 1.
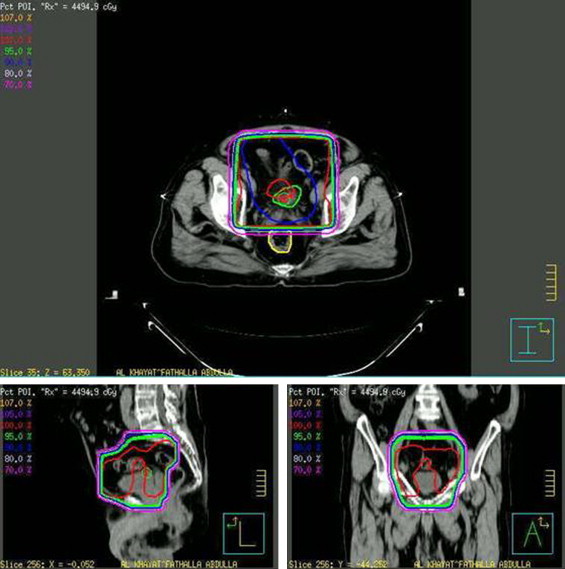
Bladder and pelvic lymphatic irradiation isodose distributions.
Fig. 2.
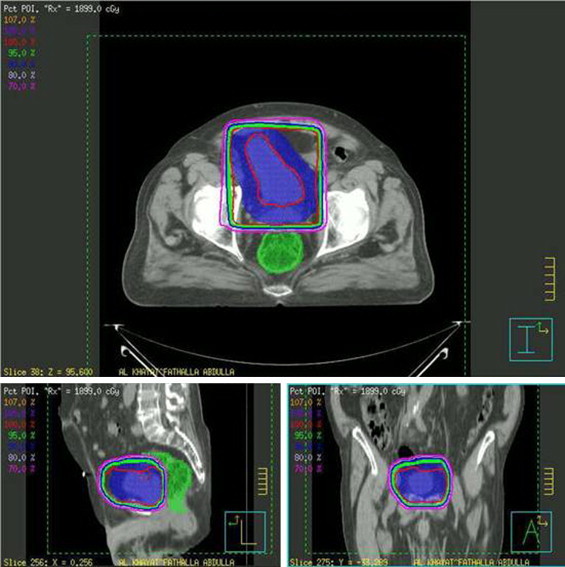
Bladder and tumor cone-down irradiation isodose distributions.
4. Results
cCRT was actively recommended as an alternative treatment to radical cystectomy in 8 patients. Three patients expressed their preference to preserve the native bladder, while in 3 patients surgery was contraindicated due to medical conditions. All patients received the scheduled induction/concurrent chemoradiation doses as planned. In all but one patient, combined-modality treatment was well tolerated (one patient developed meningitis). Nine patients exhibited WHO grade I and/or II hematological, gastrointestinal and urinary toxicity. Evaluation of local response via trans-urethral cystoscopic-guided biopsy was performed in eleven patients, of whom 8 (73%) achieved CR and 3 (27%) achieved PR. Two of the three patients who did not have cystoscopic evaluation developed distant metastases shortly after initial treatment while the third patient lost follow-up. Four out of the 8 patients who achieved CR were treated with additional bladder irradiation up to 6400 cGy. The remaining 4 patients lost follow-up. None of the patients demonstrated late toxicity at the time of the follow-up. After a median follow-up of 18.5 months (range 3–48 months); 5 of 8 (62.5%) patients with CR were alive. Local recurrence was not observed in any of the patients who achieved CR (Table 1). None of the patients who achieved PR went on to undergo salvage cystectomy.
Table 1.
Patient characteristics and outcome.
| No | Age/sex | Tumor/stage | Induction chemotherapy | Indication for bladder preservation | Concurrent chemotherapy | Pelvic RT/Gy | Bladder RT/Gy | Initial evaluation | Local recurrence | Distant recurrence | Status | F/U in months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53/M | T3bN0 | Gemcitabine Cisplatin |
Recommended by physician | Cisplatin | 45 | – | CR | No | No | Alive NED |
27 |
| 2 | 63/M | T2N0 | Gemcitabine Carboplatin |
Patient preference | Cisplatin | 45 | – | CR | No | No | Alive NED |
47 |
| 3 | 74/M | T2aN0 | Gemcitabine Carboplatin |
Patient preference | Cisplatin | 45 | 19 | CR | No | No | Alive NED |
11 |
| 4 | 71/M | T2N0 | Gemcitabine Cisplatin |
Recommended by physician | Carboplatin | 45 | 19 | CR | No | No | Alive NED |
6 |
| 5 | 61/M | T2N0 | Gemcitabine Cisplatin |
Cardiac disease | Cisplatin | 45 | 19 | CR | No | No | Alive NED |
21 |
| 6 | 61/M | T2aN0 | Gemcitabine Carboplatin |
Recommended by physician | Carboplatin | 45 | – | CR | No | No | Dead NED |
14 |
| 7 | 65/M | T2N0 | Gemcitabine Cisplatin |
Recommended by physician | Cisplatin | 45 | – | CR | No | Lung Bone |
Dead DTD |
48 |
| 8 | 61/F | T2N0 | Gemcitabine Cisplatin |
Morbid obesity | Cisplatin | 45 | 16 | CR | No | Pelvic LNs |
Dead DTD |
47 |
| 9 | 60/M | T3N0 | Gemcitabine Cisplatin |
Recommended by physician | Cisplatin | 45 | – | Not evaluated | – | – | Alive UDS |
3 |
| 10 | 56/F | T3bN0 | Gemcitabine Cisplatin |
Recommended by physician | Cisplatin | 45 | – | PR | Residual disease | Pelvic LNs |
Dead DTD |
16 |
| 11 | 63/M | T2N0 | Gemcitabine Cisplatin* |
Patient preference | Cisplatin | 45 | – | PR | Residual disease | Bone | Dead DTD |
4 |
| 12 | 49/M | T2N0 | Gemcitabine Cisplatin |
Recommended by physician | Carboplatin | 45 | – | PR | Residual disease | PALNs Bone |
Alive WD |
24 |
| 13 | 41/F | T2N0 | Gemcitabine Carboplatin |
Recommended by physician | Cisplatin | 45 | – | Not evaluated | – | Liver Lung Bone |
Dead DTD |
5 |
| 14 | 59/M | T2aN0 | Gemcitabine Cisplatin |
COPD | Cisplatin | 45 | – | Not evaluated | – | Lung | Dead DTD |
14 |
PALN, para-aortic lymph node; NED, no evidence of disease; DTD, due to disease; UDS, unknown disease status; WD, with disease; COPD, chronic obstructive pulmonary disease.
Patient could not tolerate regimen (developed meningitis).
5. Discussion
Urinary bladder cancer continues to pose a significant global health challenge. In the Western world, bladder cancer is the fourth most common malignancy in men and the eighth most common in women.6 In Jordan, cancers of the urinary bladder accounted for 69.2% of all urinary tract cancers in 2008 ranking the third and the twenty seventh most prevalent cancer among males and females, respectively, with a male to female ratio of 6.3:1. Worldwide, the most prevalent pathological subtype is transitional cell carcinoma accounting for up to 90% of cases [100,2]. Similarly, TCC constitutes over 95% of bladder cancers in Jordan.7 Muscle invasion – estimated to occur in 30% of cases – is a major prognostic factor since it requires more aggressive therapy.1,8 Radical cystectomy is the standard treatment for such patients resulting in a 5-year overall survival of about 45–60%.9,10 This holds true in the US; where radical cystectomy remains the preferred primary treatment modality for organ-confined (T2–4N0M0) disease.3 However, since bladder cancer is generally diagnosed after the age of 65, and rarely before 40 years of age, a significant number of patients are deemed unsuitable for surgery due to inoperability, advanced age, or comorbid conditions.11 Radical cystectomy, in addition, markedly affects the quality of life secondary to urinary diversion techniques and the associated rehabilitation necessary subsequent to neo-bladder construction.3 The non-continent state following urinary diversion has significant physical and psychological implications.12 Furthermore, complications following radical cystectomy include hemorrhage, infection, urinary leaks, pelvic lymphoceles, intestinal obstruction, and peritonitis.13 This previous statement was reiterated by Hautmann and collegues who reported their experience with 1000 radical cystectomies and subsequent neobladder formation.14
Therefore, several groups have explored less radical therapeutic strategies that aim at bladder preservation.4 Ideal candidates are patients with solitary T2 or T3 tumors, with no evidence of ureteral obstruction, a visibly complete transurethral tumor resection, with no evidence of pelvic lymph node metastases and adequate renal function2,15,16 ork between the urologist, medical and radiation oncologist is crucial for the successful completion of such organ-preserving strategies.2
The concept of combined administration of chemoradiation is designed to maximize the interaction between ionizing radiation and chemotherapeutic agents.3 At the radiobiological level, chemotherapy appears to act synergistically with radiotherapy by targeting cells in the radio-resistant phases of the growth cycle, leaving a population of synchronized, more radiosensitive cells.3 The basis behind such interactions includes spatial co-operation, prevention of emergence of resistant clones, hypoxic cell sensitization, hypoxic cell killing, inhibition of potentially lethal and sub-lethal radiation-induced damage repair and inhibition of cellular repopulation during fractionated radiotherapy.3 Cisplatin has been extensively investigated for its interaction with ionizing radiation.3 Specifically, cisplatin exerts its cytotoxic effects by chelating guanine residues, yielding monofunctional adducts and intra-strand or inter-strand crosslinks.3 The first phase II study reporting the concurrent use of cisplatin with pelvic radiotherapy in bladder cancer was published in 1982.17 More recently, a number of phase III clinical trials reporting patients randomized to cisplatin plus radiotherapy versus radiotherapy alone demonstrated a significant improvement in pelvic recurrence-free survival with combined treatment, in addition to improved bladder preservation, although no overall survival difference was observed.18 The proposed standard radiotherapy regimen for invasive bladder cancer is irradiation of the whole bladder and tumor with a 2-to 3-cm margin to a dose of 60–66 Gy.19
Nonetheless, there are no randomized phase III clinical trials directly comparing radical cystectomy and chemoradiotherapy in muscle-invasive bladder carcinoma.13 Comparing results from the non-randomized trials of chemoradiation with contemporary cystectomy series is fraught with difficulty secondary to the considerable discrepancy between clinical staging and pathologic staging, which broach forth as important confounding factors in contrasting results of surgery with those of bladder preservation.3,20 However, a considerable number of studies have shown that in terms of disease-specific survival radical radiotherapy results are equivalent to those for radical surgery at 5 years and 8 years.13 Recently, a retrospective cohort from the United Kingdom reported no statistical difference in all-cause survival between surgery and radiotherapy at a minimum follow-up period of 10 years.20 Thus, the use of bladder-preservation therapy for muscle-invasive TCC of the bladder is a valid alternative in selected cases with long-term efficacy comparable to radical cystectomy, with the added advantage of preserving excellent bladder function in the majority of long-term survivors.21 Prospective studies have reported encouraging quality of life evaluations for such patients.22
In Jordan, oncological healthcare is delivered via national, university and private hospitals. National hospitals are funded, managed and operated by the Ministry of Health and provide near-global low-cost primary care coverage. University hospitals, on the other hand, deliver specialized tertiary care. Private hospitals deliver fee-for-service care. The King Hussein Cancer Center is the sole comprehensive cancer center in the country. Unfortunately, several gaps hinder the provision of comprehensive quality care for cancer patients outside our institution, including inappropriate clinical practices, insufficient implementation of evidence-based medicine and unbalanced physical distribution of care providers.23 In this small series, we demonstrated the applicability of bladder-preserving combined-modality therapy in a low income-country. Unfortunately, there are no published reports assessing outcomes of radical cystectomy for bladder cancer in Jordan and, therefore, we are unable to compare our results with those of a surgery series. Nonetheless, and while acknowledging the limitations of our study including the retrospective nature, small sample size and the short follow-up period, we report a rate of complete pathological response in lieu with previously published reports. However, lifelong bladder surveillance is paramount, since only prompt salvage therapy can prevent a focus of a new or recurrent bladder cancer from disseminating.4
Conflict of interest statement
None declared.
References
- 1.Leliveld A.M., Doornweerd B.H., Bastiaannet E., Schaapveld M., de Jong I.J. Treatment and outcome in muscle invasive bladder cancer: a population-based survey. World J Urol. 2010 doi: 10.1007/s00345-010-0546-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Ott O.J., Rödel C., Weiss C. Radiochemotherapy for bladder cancer. Clin Oncol (R Coll Radiol) 2009;7:557–565. doi: 10.1016/j.clon.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood B.T., Jones G.D., Mellon J.K., Kockelbergh R.C., Steward W.P., Symonds R.P. Concomitant chemoradiotherapy for muscle-invasive bladder cancer: the way forward for bladder preservation? Clin Oncol (R Coll Radiol) 2005;(3):160–166. doi: 10.1016/j.clon.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Shipley W.U., Kaufman D.S., Zehr E. Selective bladder preservation by combined modality protocol treatment: long-term outcomes of 190 patients with invasive bladder cancer. Urology. 2002;60(1):62–67. doi: 10.1016/s0090-4295(02)01650-3. discussion 67–8. [DOI] [PubMed] [Google Scholar]
- 5.Sobin L.H., Wittekind C. 6th ed. John Wiley & Sons; Hoboken, New Jersey: 2002. TNM classification of malignant tumours. pp. 199–202. [Google Scholar]
- 6.Kirkali Z., Chan T., Manoharan M. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. doi: 10.1016/j.urology.2005.07.062. (6 Suppl 1) [DOI] [PubMed] [Google Scholar]
- 7.Matalka I., Bani-Hani K., Shotar A., Bani Hani O., Bani-Hani I. Transitional cell carcinoma of the urinary bladder: a clinicopathological study. Singapore Med J. 2008;49(10):790–794. [PubMed] [Google Scholar]
- 8.Chahal R., Sundaram S.K., Iddenden R., Forman D.F., Weston P.M., Harrison S.C. A study of the morbidity, mortality and long-term survival following radical cystectomy and radical radiotherapy in the treatment of invasive bladder cancer in Yorkshire. Eur Urol. 2003;43(3):246–257. doi: 10.1016/s0302-2838(02)00581-x. [DOI] [PubMed] [Google Scholar]
- 9.Lin C.C., Hsu C.H., Cheng J.C. Induction cisplatin and fluorouracil-based chemotherapy followed by concurrent chemoradiation for muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2009;75(2):442–448. doi: 10.1016/j.ijrobp.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Sternberg C.N. Current perspectives in muscle invasive bladder cancer. Eur J Cancer. 2002;38(4):460–467. doi: 10.1016/s0959-8049(01)00393-8. [DOI] [PubMed] [Google Scholar]
- 11.Chen W.C., Liaw C.C., Chuang C.K. Concurrent cisplatin, 5-fluorouracil, leucovorin, and radiotherapy for invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2003;56(3):726–733. doi: 10.1016/s0360-3016(03)00124-x. [DOI] [PubMed] [Google Scholar]
- 12.Large M.C., Katz M.H., Shikanov S., Eggener S.E., Steinberg G.D. Orthotopic neobladder versus Indiana pouch in women: a comparison of health related quality of life outcomes. J Urol. 2010;183(1):201–206. doi: 10.1016/j.juro.2009.08.148. [DOI] [PubMed] [Google Scholar]
- 13.Kotwal S., Choudhury A., Johnston C., Paul A.B., Whelan P., Kiltie A.E. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70(2):456–463. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Hautmann R.E., de Petriconi R.C., Volkmer B.G. Lessons learned from 1000 neobladders: the 90-day complication rate. J Urol. 2010;184(3):990–994. doi: 10.1016/j.juro.2010.05.037. quiz 1235. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman D.S., Shipley W.U., Feldman A.S. Bladder Cancer Lancet. 2009;374(9685):239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 16.Gamal El-Deen H.S. Initial results of retrospective study: preoperative transurethral excision plus chemotherapy and radiation therapy and trial of bladder preservation. J Egypt Natl Canc Inst. 2007;19(2):133–146. [PubMed] [Google Scholar]
- 17.Soloway M.S., Ikard M., Scheinberg M., Evans J. Concurrent radiation and cisplatin in the treatment of advanced bladder cancer: a preliminary report. J Urol. 1982;128(5):1031–1033. doi: 10.1016/s0022-5347(17)53330-3. [DOI] [PubMed] [Google Scholar]
- 18.Evans C, Debruyne F, Payne H, Solsona E, Teillac P, Tubaro A. Bladder cancer: management and future directions. Eur Urol Suppl 6(3). p. 365–73.
- 19.Pos F.J., Hart G., Schneider C., Sminia P. Radical radiotherapy for invasive bladder cancer: What dose and fractionation schedule to choose? Int J Radiat Oncol Biol Phys. 2006;64(4):1168–1173. doi: 10.1016/j.ijrobp.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Munro N.P., Sundaram S.K., Weston P.M. A 10-year retrospective review of a nonrandomized cohort of 458 patients undergoing radical radiotherapy or cystectomy in Yorkshire, UK. Int J Radiat Oncol Biol Phys. 2010;77(1):119–124. doi: 10.1016/j.ijrobp.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 21.Michaelson M.D., Shipley W.U., Heney N.M., Zietman A.L., Kaufman D.S. Selective bladder preservation for muscle-invasive transitional cell carcinoma of the urinary bladder. Br J Cancer. 2004;90(3):578–581. doi: 10.1038/sj.bjc.6601580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagrange J.L., Bascoul-Mollevi C., Geoffrois L. Quality of life assessment after concurrent chemoradiation for invasive bladder cancer: results of a multicenter prospective study (GETUG 97-015) Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Al-Jauissy M.S., Al-Hassan M., Akhu-Zaheya L. Healthcare needs of noninstitutionalized Jordanian cancer patients: an exploratory descriptive study. Cancer Nurs. 2009;32(4):291–298. doi: 10.1097/NCC.0b013e3181a0221e. [DOI] [PubMed] [Google Scholar]


