Abstract
Background
Adjuvant radiotherapy (RTE) still has a fundamental role as a post-operative treatment of locally advanced soft tissues sarcomas of the extremities. Moreover the employment of combined modalities in locally advanced soft tissues sarcomas of the extremities allow to maximize the chance of local cure even in difficult presentation cases, and possibly improve survival, especially in high-risk disease patients. In patients with sarcomas of the extremities in which definitive surgery has not been radical (with positive or “close” margins) radiotherapy can improve the results in terms of Disease Free Survival (DFS) and, together with chemotherapy, of Overall Survival (OS). We recommend radiotherapy in case of deep tumor location, inadequate surgical margins and grade 3 tumour; for positive or “marginal (or close)” excision (that means inadequate surgery) or in selected patients with a bad prognosis, we believe that a multidisciplinary approach can be preferable.
Introduction
Adjuvant radiotherapy (RTE) still has a fundamental role as a post-operative treatment. In patients with sarcomas of the extremities in whom definitive surgery has been or not radical (positive or “close” margins), radiotherapy with chemotherapy can improve the results in terms of Disease Free Survival (DFS) and Overall Survival (OS), while RTE alone seems to improve local control.
Materials and methods
From 1/2000 to 12/2005 we treated 34 patients affected by locally advanced sarcomas of the upper or lower extremities with radiotherapy (doses ranging from 54 to 66 Gy) and chemotherapy in 18/34 with an adjuvant scheme that consisted in Epirubicine (120 mg/m2) plus Ifosfamide (7000–9000 mg/m2).
Results
Disease Free Survival (DFS) and the Overall Survival (OS) rates were 76% and 82%, respectively. Eighteen patients developed one or more long-term side effects. Most of these complications were mild: all patients experienced only erithema, edema, local sclerosis or moderate pain.
Conclusion
Radiotherapy has an important role as a post-operative treatment also when surgery was non-radical. It improves local control more in patients with high-grade sarcomas of the extremity with positive or close margins. It is still difficult to assess the role of adjuvant chemotherapy.
Keywords: Radiotherapy, Chemotherapy, Sarcomas of extremities
1. Introduction
Soft-tissue sarcomas (STS) represent a rare and heterogeneous group of tumours, accounting for 0.7% of all cancers.1
Soft-tissue sarcomas can occur anywhere in the body, but most originate in an extremity (59%), the trunk (19%), the retroperitoneum (15%), or the head and neck (9%).2
Soft-tissue sarcomas are generally classified based on the histogenesis and/or cytomorphological features of the tumour. The most commonly used systems are the French grading and the National Cancer Institute grading. Both are 3-grade systems and are mainly based on histologic type and subtype, tumour necrosis, and mitotic activity.3
The three most important prognostic factors are grade, size and depth of the primary tumour (relative to the superficial fascia).4
Patients with high-grade lesions, large (T2) sarcomas, non-extremity subsite location, or deep tumour location, are at increased risk for disease relapse and sarcoma-specific death.5,6
Adjuvant radiotherapy (RTE) still has a fundamental role as a post-operative treatment. In patients with sarcomas of the extremities in whom definitive surgery has not been radical (positive or “close” margins), radiotherapy can improve the results in terms of Disease Free Survival (DFS) and, combined with chemotherapy, in terms of Overall Survival (OS).7–10
The purpose of this study was to determine the effect of radiation therapy on disease free and overall survival among patients with primary soft-tissue sarcomas of the extremities, above all if a surgery has not been radical.
2. Materials and methods
From 1/2000 to 12/2005 we treated 34 patients affected by locally advanced sarcomas of the upper or lower extremities. These patients were treated in the majority of cases with a radical excision (12/34), with biopsy and radical excision (11/34) or with wide excision alone (11/34), followed by radiotherapy with or without chemotherapy. All the excisions were defined as surgically radical by the surgeons that performed them. But the histological data showed “closed” margins (<0.5 cm) in 7 patients and positive margins in 5. No one had evaluable distant metastasis at the time of radiotherapy. All patients underwent radiotherapy after surgery; 18/34 patients also received chemotherapy with an adjuvant scheme that consisted in Epirubicine (120 mg/m2) plus Ifosfamide (7000–9000 mg/m2). RTE was given with ≥two 3D conformal fields using 6 Mv Photon beams or photon-electron mixed beams and 6–15 Mev electron beams as a boost with a total dose >60 Gy (ICRU) in 28 patients and less than 60 Gy (ICRU) in 6 patients (range 54–66 Gy). The 12 patients with close or positive margins received both chemotherapy and radiotherapy delivered at the dose of 66 Gy.
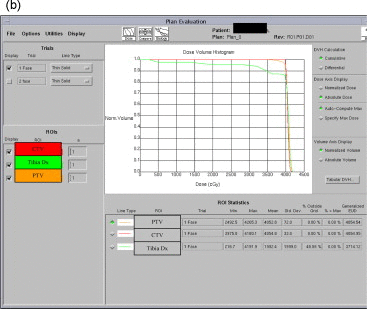
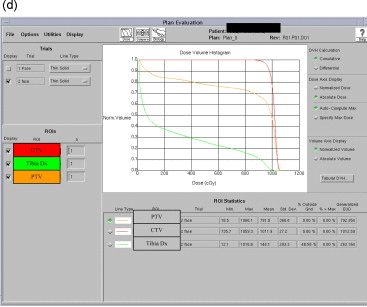
An example of treatment parameters for one patient is shown in Table 1. Table 1a represents the first part of treatment (0–50 Gy); Table 1c represents the second part of the treatment (boost) (from 50 to 60 Gy if negative margins or 64–66 Gy if “close” or positive margins). Table 1b represents the Dose Volume Histogram (DVH) of the first part of treatment (0–50 Gy) for the CTV and PTV and for the bones of the leg. Table 1d represents the Dose Volume Histogram (DVH) of the second part of treatment (from 50 to 60 Gy if negative margins or 64–66 Gy if “close” or positive margins) for the CTV and PTV and for the bones of the leg. The CTV was defined on the basis of the pre-operative MRI. The PTV was represented by the CTV plus 3 cm margins.
Table 1.
Example of treatment planning.
 |
 |
 |
 |
3. Results
All patients completed the radiotherapy course without interruption. After completion of therapy, patients were followed initially at 4-month intervals for 2 years then subsequently bi-annually for 2 years, then yearly. Surveillance was important to detect recurrences that might still be potentially curable and long-term side effects. Follow-up studies included a thorough clinical examination, routine chemistry, chest X-ray, and CT scan or magnetic resonance imaging (MRI) of the region including the primary site. With a medium follow-up of 26 months (range 12–64 months), we had 26/34 patients that were free from disease. Six patients died in a period ranging from 18 to 42 months from the end of treatment due to consequences of the progression of disease, two of them were in the group with positive margins and one in the group with close margins. Until now, one patient has had lung metastases and only 1 has had a local recurrence. These patients have been treated according to intern protocol of salvage chemotherapy. As shown in Figs. 1 and 2, the Disease Free Survival (DFS) and the Overall Survival (OS) rates were 76% and 82%, respectively.
Fig. 1.
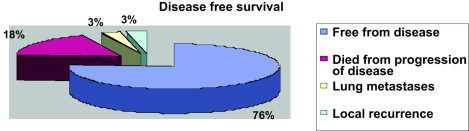
Disease free survival.
Fig. 2.
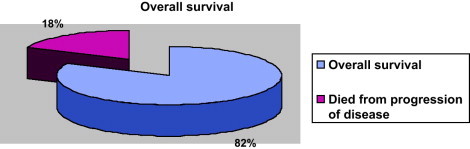
Overall survival.
Eighteen patients developed one or more long-term side effects. Most of these complications were mild: all patients experienced only a local erithema (or a different pigmentation of the skin), edema, local sclerosis or moderate pain. Five patients had mild motion impairment, particularly on the account of the lower extremity: difficulty in going up and down stairs or walking and tendency to tiredness. They did not have a significant improvement in the ability to drive a car or be employed.
No patients developed more serious complications than skin necrosis, bone fractures or sciatic nerve palsy. All patients that received chemotherapy developed haematologic toxicity: myelosuppression with grade 3–4 leukopenia was observed in 10 patients, grade 3–4 trombocytopenia in 3 and anemia requiring transfusion in 5.
4. Discussion
The employment of combined modalities in locally advanced soft-tissue sarcomas of the extremities will be able to maximize the chance of local cure even in difficult presentation, and possibly improve survival, especially in high-risk disease.
The surgical approach to soft-tissue sarcomas depends on careful pre-operative staging with MRI or CT for lesions of the extremities and a histologic diagnosis and assessment of grade. In most instances, pre-operative imaging studies allow for accurate prediction of resectability preventing the risk for local recurrence due to poor primary resections.11
Surgical resection of sarcomas with negative surgical margins remains the mainstay of treatment and allows adequate margin of normal tissue for patients with no evidence of metastatic disease, and for small low-grade superficial tumours in which adequate margins can be obtained. Surgery involves resection of all gross visible and palpable tumours en bloc, including previous biopsy scars and drain tract and large volumes of grossly normal tissue.12–14
Optimal resection seems to be the best predictive parameter for a favourable outcome in term of local control in localized STS.10
For large, deep tumours where surgical margins are likely to be close in order to preserve important neurovascular structures and bone, the addition of radiotherapy to resection has improved local control and increased limb salvage but without affecting overall survival.15 Intuitively, decreasing local failures and thus distant metastases would improve overall survival, but this has not yet been proven.
A number of retrospective reports, as well as a randomized trial from the NCI, have demonstrated that limb-sparing surgery plus post-operative irradiation produces local control rates comparable to those achieved with amputation.16,17 Five-year local control rates of 70–90%, survival rates of 70%, and limb-preservation rates of 85% can be expected. Combined limb-sparing therapy and radiation therapy is currently the standard of care for primary treatment of patients with soft-tissue sarcomas.18 Our experience with a total DFS of 76% and OS of 82% follows the tendency showed by these data.
Radiotherapy can be used as well as neoadjuvant treatment. The ideal sequence of surgery and radiation, whether to use pre-operative (neoadjuvant) or post-operative (adjuvant) irradiation for local management of soft-tissue sarcoma remains controversial on the basis of early versus late treatment complications, although pre-operative radiation can provide the best results for improved long-term function and has been addressed in retrospective as well as in prospective randomized trials.19–22 Pre-operative irradiation has the advantage of potentially rendering an unresectable tumour resectable, allowing limb-salvage surgery, reducing the risk of seeding at the time of surgery, and permitting larger radiation therapy fields without interfering with wound healing.23 However, pre-operative irradiation has also several drawbacks. They include both the inability to precisely stage patients based on pathology due to downstaging and the increased problems with wound healing.24,25 Since the pre-operative treatment is burdened by an increase in morbidity in terms of wound healing, it is deemed appropriate to apply post-operative radiotherapy and reserve the pre-operative procedure only for injuries initially not operable. This is, at the moment, the reason that led us to perform only post-operative RTE.
Unfortunately, despite major advances in surgical and radiotherapy techniques, and significant improvements in the rate of local control, ≈50% of patients will ultimately develop metastatic disease. Patients with high-risk lesions should benefit from adjuvant or neoadjuvant chemotherapy.26
It is difficult to assess the role of adjuvant chemotherapy, since a number of trials, different reviews, and meta-analysis performed in the past 20–30 years have shown conflicting results as they included small numbers of patients, with different tumour histologies, grade and location, treated with different drugs at different doses.27 Adjuvant chemotherapy has been shown to reduce the risk for local relapse, distant relapse and to improve disease free survival and quality of life. Some studies have arrived at conflicting conclusions regarding the influence of adjuvant chemotherapy on overall survival: there are overall survival benefits for specific histological types, but this is a relatively small subgroup.28–31
Adjuvant doxorubicin/ifosfamide combinations may improve relapse-free survival in carefully selected patients and can be considered for the treatment of those with tumour size >5 cm, deep tumour location, and high histologic grade.32–34
Pre-operative chemotherapy should be considered for fit, high-risk patients after a discussion of the risks and potential benefits. Retrospective review showed that neoadjuvant chemotherapy was feasible and did not compromise subsequent treatment surgery with or without radiotherapy, and did not increase post-operative morbidity in patients with soft-tissue sarcomas.36–38
The specific regimens employed have evolved over the years but generally contain both an anthracycline and ifosfamide, their use featured by the occurrence of cardiac and renal toxicity. Older patients, especially those with cardiac or renal disease, are not optimal candidates for such treatment.
Despite the theoretical advantages, there is no evidence that neoadjuvant chemotherapy is better than the adjuvant approach in terms of DFS (Disease Free Survival), and OS (Overall Survival), but response to neoadjuvant treatment is a prognostic factor for local disease control.42
Consequently, treatment depends on the specific type of sarcoma. Because sarcomas are relatively uncommon, yet comprise a wide variety of different entities, evaluation by oncology teams who have expertise in the field is recommended.
Our data revealed the clinical benefits from chemotherapy in combination with surgery and radiotherapy, although no treatment-related conclusions can be made. In fact, we cannot conclude, with our data, that the combination of chemotherapy and radiotherapy is the choice modality for these patients instead of radiotherapy alone, particularly if local control is the primary aim. But all the patients treated with chemotherapy were classified at high risk of distant metastasis; this was the motivation for the systemic therapy.
Radiotherapy has an important role as a post-operative treatment, also when non-radical. It improves local control more in patients with high-grade sarcomas of the extremity with positive or close margins.8,9 In our study we decided to give a high dose RTE to the category of patients that we considered at risk (12 patients totally); they received also chemotherapy. Finally, in terms of DFS and OS we obtained a result that is similar to results of patients treated with a really radical surgery. Obviously, the number of patients that we have treated is too small, but we believe that a high dose radiotherapy plus chemotherapy treatment protocol can give these unfortunate patients similar surviving chances. Our data are in fact similar to those that can be found in literature on this pathology.
We recommend radiotherapy in case of deep tumour location, inadequate surgical margins and grade 3 tumour; for positive or “marginal (or close)” excision (that means inadequate surgery) or in selected patients with a bad prognosis, we believe that a multidisciplinary approach can be preferable.
5. Conclusion
Surgery is the leading procedute in the treatment of soft tissue sarcomas of the extremities. Radiotherapy has an important role as a post-operative treatment above all when it was non radical. It improve local control in patients with high-grade sarcomas with positive or close margins. In our experience we decided to give a high dose RTE in this category of patients that we considered at risk of local recurrence ad distant metastases; they received also chemotherapy. Finally we obtained a result in terms of DFS and OS that is similar to those that were treated with a really radical surgery. Obviously the number of patients that we have treated is too small, but we believe that a treatment protocol based on high dose radiotherapy plus chemotherapy can give to these unfortunate patients similar surviving chances. Our data are in fact similar to those of literature for this pathology. We need further follow up in order to confirm our data.
References
- 1.Adigun I.A., Rahman G.A. A review of soft tissue sarcoma. Niger J Med. 2007;16(2):94–101. doi: 10.4314/njm.v16i2.37289. [DOI] [PubMed] [Google Scholar]
- 2.Cormier J.N., Pollock R. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 3.Coindre J.M. Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med. 2006;130(10):1448–1453. doi: 10.5858/2006-130-1448-GOSTSR. [DOI] [PubMed] [Google Scholar]
- 4.Skubitz K.M., D’Adamo DR:, Sarcoma Mayo Clin Proc. 2007;82(11):1409–1432. doi: 10.4065/82.11.1409. [DOI] [PubMed] [Google Scholar]
- 5.Stefanovski P.D., Bidoli E., De Paoli A., Buonadonna A., Boz G., Libra M. Prognostic factors in soft tissue sarcomas: a study of 395 patients. Eur J Surg Oncol. 2002;28(2):153–164. doi: 10.1053/ejso.2001.1242. [DOI] [PubMed] [Google Scholar]
- 6.Eilber F.C., Brennan M.F., Riedel E., Alektiar K.M., Antonescu C.R., Singer S. Prognostic factors for survival in patients with locally recurrent extremity soft tissue sarcomas. Ann Surg Oncol. 2005;12(3):228–236. doi: 10.1245/ASO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Strander H., Turesson I., Cavallin-Ståhl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol. 2003;42(5–6):516–531. doi: 10.1080/02841860310014732. [DOI] [PubMed] [Google Scholar]
- 8.Alektiar K.M., Velasco J., Zelefsky M.J., Woodruff J.M., Lewis J.J., Brennan M.F. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2002;48(4):1051–1058. doi: 10.1016/s0360-3016(00)00753-7. [DOI] [PubMed] [Google Scholar]
- 9.Jebsen N.L., Trovik C.S., Bauer H.C., Rydholm A., Monge O.R., Hall K.S. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: a Scandinavian Sarcoma Group Study. Int J Radiat Oncol Biol Phys. 2008;71(4):1196–1203. doi: 10.1016/j.ijrobp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Khanfir K., Alzieu L., Terrier P., Le Péchoux C., Bonvalot S., Vanel D. Does adjuvant radiation therapy increase loco-regional control after optimal resection of soft-tissue sarcoma of the extremities? Eur J Cancer. 2003;39(13):1872–1880. doi: 10.1016/s0959-8049(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 11.Tzeng C.W., Smith J.K., Heslin M.J. Soft tissue sarcoma: preoperative and postoperative imaging for staging. Surg Oncol Clin N Am. 2007;16(2):389–402. doi: 10.1016/j.soc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Röhrborn A., Röher H.D. Surgical aspects in the multidisciplinary treatment of soft tissue sarcomas. Schweiz Rundsch Med Prax. 1998;87(34):1050–1060. [PubMed] [Google Scholar]
- 13.Clarkson P., Ferguson P.C. Primary multidisciplinary management of extremity soft tissue sarcomas. Curr Treat Options Oncol. 2004;5(6):451–462. doi: 10.1007/s11864-004-0034-2. [DOI] [PubMed] [Google Scholar]
- 14.DeLaney T.F. Optimizing radiation therapy and post-treatment function in the management of extremity soft tissue sarcoma. Curr Treat Options Oncol. 2004;5(6):463–476. doi: 10.1007/s11864-004-0035-1. [DOI] [PubMed] [Google Scholar]
- 15.Schlieman M., Smith R., Kraybill W.G. Adjuvant therapy for extremity sarcomas. Curr Treat Options Oncol. 2006;7(6):456–463. doi: 10.1007/s11864-006-0021-x. [DOI] [PubMed] [Google Scholar]
- 16.Yang J.C., Chang A.E., Baker A.R., Sindelar W.F., Danforth D.N., Topalian S.L. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 17.Swallow C.J., Catton C.N. Local management of adult soft tissue sarcomas. Semin Oncol. 2007;34(3):256–269. doi: 10.1053/j.seminoncol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson P.C. Surgical considerations for management of distal extremity soft tissue sarcomas. Curr Opin Oncol. 2005;17(4):366–369. doi: 10.1097/01.cco.0000166651.38417.c8. [DOI] [PubMed] [Google Scholar]
- 19.Wunder J.S., Nielsen T.O., Maki R.G., O'Sullivan B., Alman B.A. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. 2007;8:513–524. doi: 10.1016/S1470-2045(07)70169-9. [DOI] [PubMed] [Google Scholar]
- 20.Cannon C.P., Ballo M.T., Zagars G.K., Mirza A.N., Lin P.P., Lewis V.O. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006;107(10):2455–2461. doi: 10.1002/cncr.22298. [DOI] [PubMed] [Google Scholar]
- 21.Davis A.M., O'Sullivan B., Turcotte R., Bell R., Catton C., Chabot P. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75(1):48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Wolfson A.H. Preoperative vs postoperative radiation therapy for extremity soft tissue sarcoma: controversy and present management. Curr Opin Oncol. 2005;17(4):357–360. doi: 10.1097/01.cco.0000161745.24887.82. [DOI] [PubMed] [Google Scholar]
- 23.Zagars G.K., Ballo M.T., Pisters P.W., Pollock R.E., Patel S.R., Benjamin R.S. Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys. 2003;56(2):482–488. doi: 10.1016/s0360-3016(02)04510-8. [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan B., Davis A.M., Turcotte R., Bell R., Catton C., Chabot P. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. The Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 25.Davis A.M., O'Sullivan B., Bell R.S., Turcotte R., Catton C.N., Wunder J.S. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20(22):4472–4477. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 26.Kasper B. Standards and novel therapeutic options in the treatment of patients with soft tissue sarcoma. Rev Recent Clin Trials. 2007;2(3):206–211. doi: 10.2174/157488707781662706. [DOI] [PubMed] [Google Scholar]
- 27.Tierney J.F., Mosseri V., Stewart L.A., Souhami R.L., Parmar M.K. Adjuvant chemotherapy for soft-tissue sarcoma: review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995;72(2):469–475. doi: 10.1038/bjc.1995.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bramwell V., Rouesse J., Steward W., Santoro A., Schraffordt-Koops H., Buesa J. Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma—reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1994;12(6):1137–1149. doi: 10.1200/JCO.1994.12.6.1137. [DOI] [PubMed] [Google Scholar]
- 29.Frustaci S., Gherlinzoni F., De Paoli A., Bonetti M., Azzarelli A., Comandone A. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1235–1237. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 30.Cormier J.N., Huang X., Xing Y., Thall P.F., Wang X., Benjamin R.S. Cohort analysis of patients with localized, high-risk, extremity soft tissue sarcoma treated at two cancer centers: chemotherapy-associated outcomes. J Clin Oncol. 2004;22(22):4567–4574. doi: 10.1200/JCO.2004.02.057. [DOI] [PubMed] [Google Scholar]
- 31.Singer S., Demetri G., Baldini E., Fletcher C. Management of soft-tissue sarcomas: an overview and update. Lancet Oncol. 2000;1:75–85. doi: 10.1016/s1470-2045(00)00016-4. [DOI] [PubMed] [Google Scholar]
- 32.Frustaci S., De Paoli A., Bidoli E., La Mura N., Berretta M., Buonadonna A. Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology. 2003;65(Suppl. 2):80–84. doi: 10.1159/000073366. [DOI] [PubMed] [Google Scholar]
- 33.Maki R.G. Role of chemotherapy in patients with soft tissue sarcomas. Expert Rev Anticancer Ther. 2004;4(2):229–236. doi: 10.1586/14737140.4.2.229. [DOI] [PubMed] [Google Scholar]
- 34.Röper B., Licht T. Soft tissue sarcoma of the extremities: current state of the art of adjuvant therapy. MMW Fortschr Med. 2006;148(17):32–36. [PubMed] [Google Scholar]
- 36.Gortzak E., Azzarelli A., Buesa J., Bramwell V.H., van Coevorden F., van Geel A.N. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37(9):1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 37.Meric F., Milas M., Hunt K.K., Hess K.R., Pisters P.W., Hildebrandt G. Impact of neoadjuvant chemotherapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol. 2000;18(19):3378–3383. doi: 10.1200/JCO.2000.18.19.3378. [DOI] [PubMed] [Google Scholar]
- 38.Blay J.Y., Bonvalot S., Fayette J., Stockle E., Ray-Coquard I., Coindre J.M. Neoadjuvant chemotherapy in sarcoma. Bull Cancer. 2006;93(11):1093–1098. [PubMed] [Google Scholar]
- 42.Eilber F.C., Rosen G., Eckardt J., Forscher C., Nelson S.D., Selch M. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–3209. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]


