Abstract
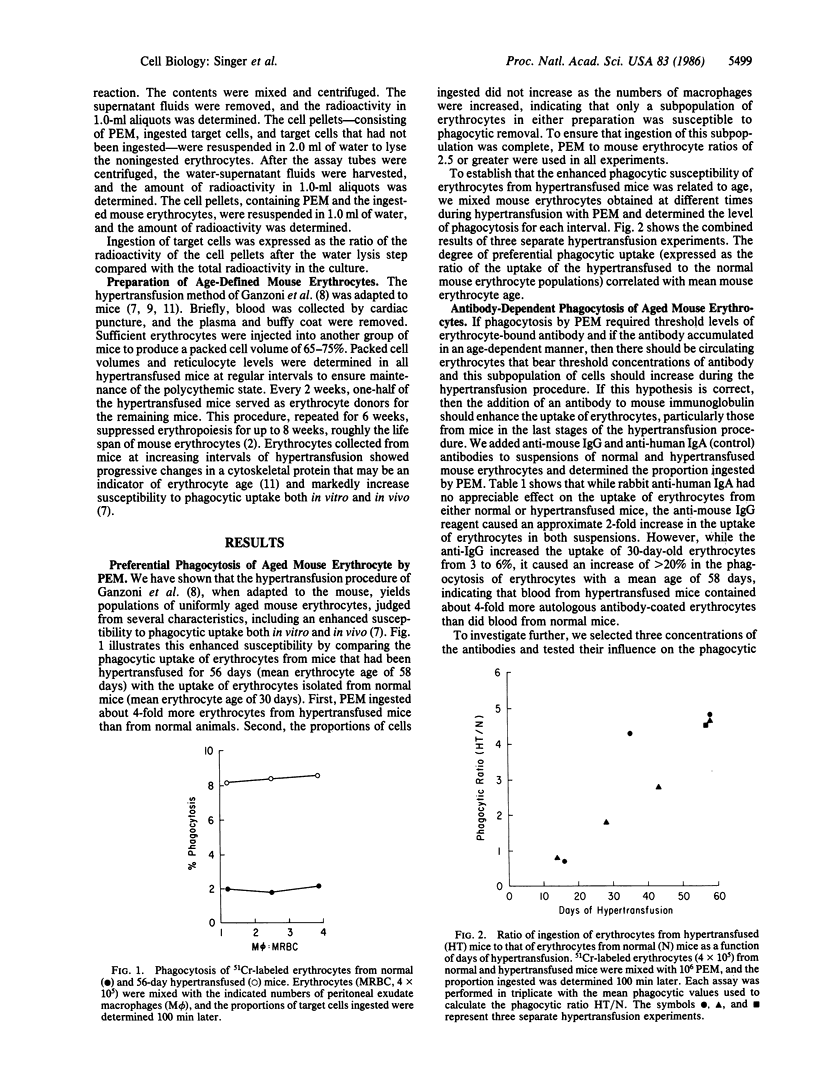
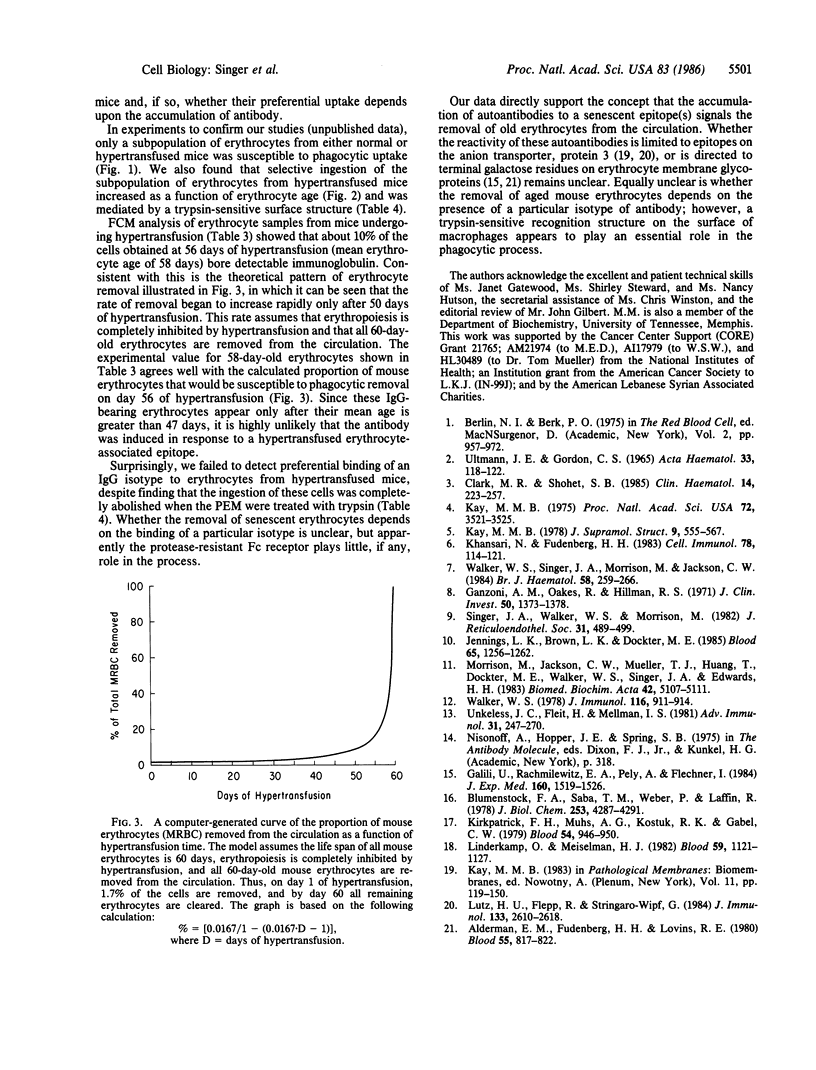
We tested the hypothesis that the accumulation of bound autologous antibody on a "senescent epitope" identifies aged erythrocytes for phagocytic removal by macrophages. Erythrocytes were collected from mice maintained on a hypertransfusion protocol designed to yield cells of defined age. The mouse erythrocytes were assayed for the presence of bound antibody by measuring their susceptibility to ingestion by macrophages from mouse peritoneal exudates and by flow cytofluorometry. Both assays disclosed that only the oldest mouse erythrocytes bore detectable levels of antibody. Flow cytofluorometric analysis revealed that the frequency distribution of IgG isotypes bound to the cells reflected their levels in normal serum. Finally, treatment with trypsin abolished the ability of the macrophages to ingest erythrocytes aged in vivo. These findings support the hypothesis that antibody mediates the clearance of senescent mouse erythrocytes from the circulation and demonstrate that the presence of a trypsin-sensitive recognition structure on macrophages is an essential requirement in this homeostatic process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman E. M., Fudenberg H. H., Lovins R. E. Binding of immunoglobulin classes to subpopulations of human red blood cells separated by density-gradient centrifugation. Blood. 1980 May;55(5):817–822. [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Clark M. R., Shohet S. B. Red cell senescence. Clin Haematol. 1985 Feb;14(1):223–257. [PubMed] [Google Scholar]
- Galili U., Rachmilewitz E. A., Peleg A., Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984 Nov 1;160(5):1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzoni A. M., Oakes R., Hillman R. S. Red cell aging in vivo. J Clin Invest. 1971 Jul;50(7):1373–1378. doi: 10.1172/JCI106619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L. K., Brown L. K., Dockter M. E. Quantitation of protein 3 content of circulating erythrocytes at the single-cell level. Blood. 1985 May;65(5):1256–1262. [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Khansari N., Fudenberg H. H. Phagocytosis of senescent erythrocytes by autologous monocytes: requirement of membrane-specific autologous IgG for immune elimination of aging red blood cells. Cell Immunol. 1983 May;78(1):114–121. doi: 10.1016/0008-8749(83)90264-2. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick F. H., Muhs A. G., Kostuk R. K., Gabel C. W. Dense (aged) circulating red cells contain normal concentrations of adenosine triphosphate (ATP). Blood. 1979 Oct;54(4):946–950. [PubMed] [Google Scholar]
- Linderkamp O., Meiselman H. J. Geometric, osmotic, and membrane mechanical properties of density-separated human red cells. Blood. 1982 Jun;59(6):1121–1127. [PubMed] [Google Scholar]
- Lutz H. U., Flepp R., Stringaro-Wipf G. Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 protein, the major integral membrane protein of human red blood cells. J Immunol. 1984 Nov;133(5):2610–2618. [PubMed] [Google Scholar]
- Singer J. A., Walker W. S., Morrison M. Interaction of a mouse macrophage cell line with homologous erythrocytes. J Reticuloendothel Soc. 1982 Jun;31(6):489–499. [PubMed] [Google Scholar]
- ULTMANN J. E., GORDON C. S. LIFE SPAN AND SITES OF SEQUESTRATION OF NORMAL ERYTHROCYTES IN NORMAL AND SPLENECTOMIZED MICE AND RATS. Acta Haematol. 1965 Feb;33:118–126. doi: 10.1159/000209520. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Walker W. S. Separate Fc-receptors for immunoglogulins IgG2a and IgG2b on an established cell line of mouse macrophages. J Immunol. 1976 Apr;116(4):911–914. [PubMed] [Google Scholar]
- Walker W. S., Singer J. A., Morrison M., Jackson C. W. Preferential phagocytosis of in vivo aged murine red blood cells by a macrophage-like cell line. Br J Haematol. 1984 Oct;58(2):259–266. doi: 10.1111/j.1365-2141.1984.tb06084.x. [DOI] [PubMed] [Google Scholar]


