Abstract
Aim
Review of literature and adjuvant treatment in Hemangiopericytoma after complete resection.
Background
Intracranial hemangiopericytoma (HPC) is an uncommon malignant vascular tumor arising from mesenchymal cells with pericytic differentiation. Surgery remains the mainstay treatment, and adjuvant radiation therapy appears to be appropriate for patients with high grade tumors or incomplete resection. We present our experience and review of the literature.
Materials and methods
We describe two cases of intracranial hemangiopericytoma located in the frontal lobe of the CNS. Both patients underwent complete tumor resection followed by adjuvant fractionated radiotherapy and completed treatment without interruptions.
Results
A local recurrence was observed in one of these cases and fractionated stereotactic radiotherapy was performed. Both patients are alive and disease has been under control up to date.
Conclusion
The treatment of choice for intracranial hemangiopericytoma is a complete surgical resection as long as possible. Adjuvant radiotherapy of HPC can result in increased tumor control and should be considered as an effective treatment for patients with high grade or demonstrated residual tumor in the postoperative period. Salvage treatment using limited-field fractionated radiotherapy for local recurrence treatment is considered an acceptable option.
Keywords: Hemangiopericytoma, Radiotherapy, Fractionated radiotherapy, Stereotactic radiosurgery
1. Background
Intracranial hemangiopericytoma (HPC) is an infrequent tumor with a high tendency to recur and to metastasize outside the central nervous system (CNS). We present two cases of Hemangiopericytomas located in the frontal lobe of the CNS; both patients underwent surgery with complete resection of tumour followed by an adjuvant fractionated radiotherapy. A Clinical and radiographic follow up are presented. One with subsequent relapse and salvage radiotherapy. A review of the literature was performed to conclude that a limited-field fractionated radiotherapy appears to represent a reasonable approach for postsurgery initial treatment of HPCs in contrast to other focal techniques.
2. Aim
Treatment of hemangiopericytoma represents a challenging situation for radiation oncologists. Lack of the literature and uncertainty of what to do in case of relapse are two aspects that make these treatments very difficult to decide upon. We present our experience and review of the literature as an example in these critical situations.
3. Introduction
Hemangiopericytomas (HPCs) of the central nervous system are rare highly cellular and vascularized mesenchymal tumors exhibiting a characteristic monotonous low-power appearance and a well-developed, variably thick-walled, branching “staghorn” vasculature. They constitute <1% of all intracranial tumors and are almost always attached to the Dura. HPCs correspond histologically to WHO grade II, with anaplastic HPCs corresponding to WHO grade III, and at present meningeal HPC is recognized as a clinicopathologically well-characterized malignancy distinct from meningioma. HPC cells are diffusely immunoreactive for vimentin (85%), CD34 (30–100%) and negative S-100 protein.1
HPCs tend to occur at a younger age than meningiomas (the mean age at diagnosis, 43 years), and more often in men than in women (male/female ratio: 1.4:1).2,3
The symptoms depend on where they are located; it usually appears with symptoms of intracranial hypertension (headache, vomiting), less frequently seizures or focal neurological deficits. The average duration of symptoms before diagnosis ranges from 3 to 6 months.4
Regarding the radiological features, it is virtually impossible to make a differential diagnosis between meningiomas and HPC based only on images.5,6 Both conditions are often associated with the meninges (dura mater) and show a very similar intracranial distribution, however, bone erosion, the existence of large vessels within the lesion, and brain edema are typical of the HPCs. In the vast majority of published series they have been described as single lesions.5–7
As for treatment, the choice is radical excision followed by postoperative radiotherapy (RTP);2,7–9,11,13
Despite appropriate treatment, HPC exhibits a high tendency for recurrence.11 Early metastases are rare, and increase over time, reaching 64% at 15 years from the initial approach.2
Overall survival of patients with HPC ranges from 84 to 144 months in most series7,8,2 and is strongly influenced by the follow-up time: at 5 years it is 67%, at 10 years, 40% and at 15 years, 23%.2
We present our single institution experience in these types of tumors discussing fractionation, radiation techniques and the role of radiotherapy.
4. Materials and methods
We retrospectively reviewed the treatment outcomes, patterns of disease recurrence, and role of radiotherapy in two patients diagnosed with HPCs treated with total radical excision followed by postoperative radiotherapy at the Institut Català d’Oncologia (Spain).
5. Case A
A 72-year-old woman with no previous medical history of interest, presented a partial seizure in April 2003. A cranial computed tomography (CT) was performed showing the presence of a bilateral frontal expansive lesion, well-defined limits with heterogeneous contrast captures administration (Fig. 1). These findings were confirmed by MRI. Surgery was the initial treatment, and a complete removal of the tumor was performed. No evidence of further tumor after immediate postoperative CT was detected. Fractionated external beam radiation therapy was prescribed. A three-dimensional (3D) limited-field planning was used with two opposite and parallel lateral fields using a Varian Clinac® Linear Accelerator 600. The patient was immobilized with a thermoplastic head and neck mask for precise repositioning. Fractionation schedule was 2 Gy/session, five times a week until a total dose of 60 Gy.
Fig. 1.

A-B CT axial view. Bifrontal tumor at diagnosis.
After 43 months of follow up, the patient being asymptomatic, tumor recurrence was found with a lesion of 1 cm near the prior tumorectomy region (Fig. 2). Surgical rescue was ruled out by the tumor board, indicating stereotactic fractionated radiotherapy.
Fig. 2.
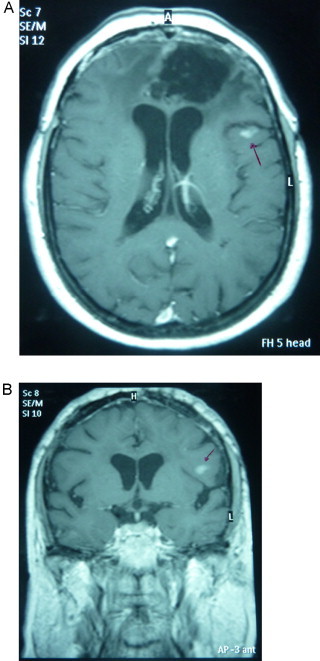
A-B IMR axial and coronal view of local recurrence.
The recurrence was treated in January 2007 using a Varian Clinac® 600 stereotactic system with a BrainLAB mask. A total dose of 25 Gy was delivered in 5 Gy per fraction with 6-mV photon. Gross target volume (GTV) was outlined according to MRI images, and a 5-mm margin was added to define CTV plus 2 mm to perform the planning target volume (PTV). Critical organs and structures including the optic chiasm, brainstem, eyes and left optic nerve were contoured. Dosimetric characteristics are shown in Table 1 and Fig. 3.
Table 1.
Treatment characteristics.
| Parameter | Measurement |
|---|---|
| GTV volume | 0.3 cm3 |
| PTV volume | 1.09 cm3 |
| Max. dose PTV | 26.5 Gy |
| Min. dose PTV | 24.0 Gy |
| Dosimetry specifications | |
|---|---|
| Number isocenter | 1 |
| Number beams | 5 |
| Dose prescribed (100%)/fraction (Gy) | 5 |
| Dose prescribed (100%) total (Gy) | 25 |
GTV, gross target volume; PTV, planned target volume; Max., maximum; Min., minimum.
Fig. 3.
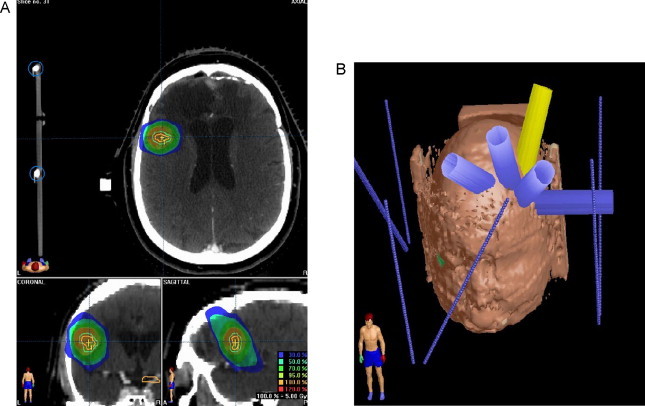
Treatment plan and dosimetric characteristics. (A) Dosimetric characteristics in relapse area and (B) treatment plan.
6. Case B
A 48-year-old man presented with a 2 year history of progressive anosmia, decreased visual acuity and changes in mood. MRI images revealed a large bilateral frontal tumor thought to be a meningioma preoperatively.
In October 2009 the patient underwent a bicoronal craniotomy gross total resection of the tumor.
The microscopic examination revealed tumor cells immunoreactive to Vimentin, CD34 and CD99, and negative to S-100 protein, confirming the diagnosis of hemangiopericytoma (WHO grade II) (Fig. 4).
Fig. 4.

Tumor with marked cellularity and monotonous appearence of the tumor cells. The cells have oval nuclei with scant cytoplasm. A prominent vascular network is noted with thin walled vessels with a “staghorn” appearance. H&E 20×.
Following surgery using a Varian Clinac® 600, 6-mV photon, the patient underwent three-dimensional limited-field planning fractionated radiotherapy with a total dose of 54 Gy at 1.8 Gy/daily fraction. GTV was outlined according to MRI images fusion, and a 2-cm margin was added to define the clinical target volume (CTV), the PTV was defined as more than 0.5-cm from the CTV. Critical organs and structures were contoured.
Dosimetric characteristics are shown in Table 2 and Fig. 5.
Table 2.
Treatment characteristics.
| Parameter | Measurement |
|---|---|
| GTV volume | 83.1 cm3 |
| CTV volume | 282.0 cm3 |
| PTV volume | 427.7 cm3 |
| Max. dose PTV | 104.9% |
| Min. dose PTV | 57.3% |
| Mean dose PTV | 99.8% |
| Number isocenter | 1 |
| Number beams | 3 |
| Dose prescribed (=100%) | 54.0 Gy |
| Dose fraction | 1.8 Gy |
GTV, gross target volume; PTV, planned target volume; Max., maximum; Min., minimum.
Fig. 5.
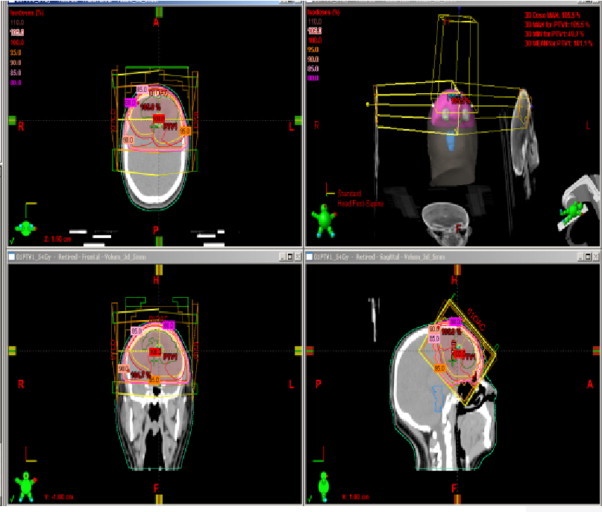
Treatment plan and dosimetrics characteristics.
7. Results
Limited-field radiotherapy was well tolerated by the patients and completed without interruptions. In both cases, acute toxicity was mild and included skin erytema GI and partial alopecia GII. To date, no severe late effects have been observed.
The range of doses applied for the initial treatment after the surgery was between 54 and 60 Gy 1.8–2.0/day.
In the Case A, local recurrence was observed at 43 months after initial treatment of surgery followed by radiotherapy. Control of initial symptoms was achieved. Reirradiation (fractionated stereotactic radiotherapy) was applied, obtaining a radiological control in the last follow up. Currently, the patient is alive with a progression-free survival of 48 months.
In Case B, the patient is 9 months after postoperative adjuvant radiotherapy, currently, with improvement of initial symptoms and no radiological signs of recurrence.
8. Discussion
Given the low incidence of HPCs, no multi-institutional prospective trials can be found to establish standards in adjuvant radiotherapy.
The attempt to complete surgical excision whenever possible has remained a standard primary approach in intracranial HPCs, some authors showing that the extent of resection is a major factor in local control of this disease.10,11,13 However, even after gross-total resection, recurrence is common.11 Guthiere et al.2 reported recurrence at a mean of 47 months, the overall disease recurrence-free (DRF) rates 5 and 10 years after initial surgery were 65% and 76%, respectively. Alen et al.7 reported a mean time to disease recurrence of 68 months.
Because of the high rate of recurrence, radiotherapy has been proposed for postsurgical initial treatment of HPCs, even when a gross-total resection has been achieved.2,11,14,15
The role of radiotherapy in HPCs has proven useful for local control of the disease and increase in the recurrence-free interval.2–9 Gutherie et al.2 demonstrated the role of postoperative radiotherapy for intracranial HPCs. Among the 44 cases described in their series, patients receiving adjuvant radiotherapy had improved disease-free survival (mean of 75 versus 34 months, p < 0.05), although overall survival was not statistically different, adjuvant radiotherapy extended the survival period from 62 months to 92 months. Dufour et al.9 also found in their series that local disease recurrence rates for patients treated with surgery followed by radiotherapy and patients treated with surgery alone were 12% and 88%, respectively, and showed that postoperative radiotherapy provided benefit with regard to local disease recurrence. Kim et al.13 did not find adjuvant radiotherapy to have a statistically significant effect on local control, yet they found that 5-year disease recurrence-free survival rates associated with complete excision alone and complete excision with adjuvant radiotherapy were 70% and 100%, respectively. More recently, Soyuer et al.16 reported 5-, 10- and 15-year overall survival rates of 85%, 68%, and 43%, respectively, in a series of 29 patients in which 34% underwent surgery followed by adjuvant radiotherapy, which is comparable with the 5- and 10-year survival rates reported by Guthiere et al.2 Table 3.
Table 3.
Intracranial hemangiopericytoma series.
| Reference | No of cases | CS% | PS% | Adjuvant RT% | LR% | OS/5 years% |
|---|---|---|---|---|---|---|
| Guthiere et al. 2 | 44 | 48 | 39 | 39 | 66 | 67 |
| Alen et al. 7 | 12 | 83 | 17 | 42 | 33 | N/A |
| Dufour et al. 9 | 17 | 76 | 24 | 47 | 52 | N/A |
| Kim et al. 13 | 31 | 77 | 23 | 35 | 39 | 96 |
CS, complete surgery; PS, partial surgery; RT, radiotherapy; LR, local recurrence; OS, overal survival.
One of the longest follow-up periods (mean follow-up of 123 m) in hemangioperycitoma has been published by Schiariti et al.18. This group found than in patients with a complete resection who underwent adjuvant external beam radiotherapy (EBRT) the mean recurrence-free interval was 126.3 months longer than if they did not receive EBRT, also overall survival was 126 months longer if the received EBRT.
EBRT is the irradiation technique recommended for the adjuvant treatment of HPCs, dose not less than 50 Gy or more, usually between 50 and 60 Gy at 1.8–2.0 Gy/day.2–9
Besides the tendency to recur locally, the HPC also recur throughout the neuraxis, and unlike the majority of primary intracranial tumors, metastasizes to extraneural sites, several years later and despite adequate treatment. Most commonly to the bone, liver and lungs.2,13,15 This determines the importance of adequate follow-up in long term.
Stereotactic radiosurgery (SRS) is considered an important management option for patients with recurrent or residual intracranial HPCs.10–12 Sheehan et al.10 demonstrated in their series of 14 patients with recurrent HPC treated with SRS that at doses >15 Gy (single dose) local control can be achieved in 80% percent of cases. Kano et al.12 with the same single dose show a progression-free survival (PFS) rate at 5 years was 89% in recurrent intracranial HPCs; in addition, they found that the factors associated with improved PFS included lower grade and higher marginal dose. More recently, Kim et al.17 analyzed retrospectively the efficacy of SRS in the treatment of recurrent intracranial HPCs, achieving similar results to those reported in other series; however, they demonstrated that doses higher than previously used (around 15 Gy) are desirable for better local tumor control of HPCs without severe adverse radiation effects. The mean tumor volume was 2.2 cm3 and the mean marginal dose was 18 Gy, the actuarial local control tumor rates at 1, 2 and 5 years were 100%, 84.6% and 67.7%, respectively. SRS provides an effective and safe adjuvant management option for patients with recurrent or residual HPCs.
9. Conclusion
HPCs have a high risk of developing disease recurrence, and represents a major problem. The treatment of choice for intracranial hemangiopericytoma is a complete surgical resection whenever technically possible. A collective analysis of published reports, supports the role of postoperative radiotherapy in the management of intracranial HPCs. In fact, adjuvant radiotherapy appears to be a therapeutic factor most related to the final outcome, specially if it is preceded by a total excision of the tumor. Radiosurgery has been shown to be an effective treatment for recurrent HPCs.
Although doses above 50 Gy are necessary to control local disease, given the low incidence of these tumors, there is no standard treatment guidelines based on evidence that support the choice of a specific type of radiation treatment technique.
In our experience, salvage treatment using limited-field fractionated radiotherapy of local recurrence was implemented, obtaining good results in terms of progression-free survival.
Overall and progression-free survival times of patients with HPCs have been improved with time. However, there is no specific treatment of HPC, which remains a great challenge for oncologists to research and publish their experience with new cases so as to provide us with scientific support and bases for specific treatment of these types of tumors.
Conflict of interest
None declared.
References
- 1.Giannini C., Rushing E.J., Hainfellner J.A. Hemangiopericytoma. 3rd ed. 2007. Who classification of tumours of the central nervous system. pp. 178–180. [Google Scholar]
- 2.Guthiere B.L., Ebersold M.J., Scheithauer B.W. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514–522. [PubMed] [Google Scholar]
- 3.Jellinger K., Paulus W., Slowik F. The enigma of meningeal hemangiopericytoma. Brain Tumor Pathol. 1991;8:33–43. [Google Scholar]
- 4.Fountas K.N., Kapsalaki E., Kassam M., Feltes C.H., Dimopoulos V.G., Robinson J.S., Smith J.R. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006;29:145–153. doi: 10.1007/s10143-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 5.Sibtain N.A., Butt S., Connor S.E.J. Imaging features of central nervous system haemangiopericytomas. Eur Radiol. 2007;17:1685–1693. doi: 10.1007/s00330-006-0471-3. [DOI] [PubMed] [Google Scholar]
- 6.Jalali R., Srinivas C., Nadkarni D., Rajasekharan P. Suprasellar haemangiopericytoma-challenges in diagnosis and treatment. Acta Neurochir (Wien) 2008;150:67–71. doi: 10.1007/s00701-007-1474-9. [DOI] [PubMed] [Google Scholar]
- 7.Alen J.F., Lobato R.D., Gómez P.A., Boto G.R., Lagares A., Ramos A., Ricoy J.R. Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir (Wien) 2001;143:575–586. doi: 10.1007/s007010170062. [DOI] [PubMed] [Google Scholar]
- 8.Mena H., Rivas J.L., Pezeshkpour G.H., Cowan D.N., Parisi J.E. Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol. 1991;22:84–91. doi: 10.1016/0046-8177(91)90067-y. [DOI] [PubMed] [Google Scholar]
- 9.Dufour H., Metellus P., Fuentes S., Murracciole X., Regis J., Figarella-Branger D., Grisoli F. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. 2001;48:756–762. doi: 10.1097/00006123-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan J., Kondziolka D., Flickinger J., Lunsford L.D. Radiosurgery for treatment of recurrent intracranial hemangiopericytomas. Neurosurgery. 2002;51:905–911. doi: 10.1097/00006123-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Soyuer S., Chang E.L., Selek U., McCutcheon I.E., Maor M.H. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004;100:1491–1497. doi: 10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 12.Kano H., Niranjan A., Kondziolka D., Flickinger J.C. Adjuvant stereotactic radiosurgery after resection of intracraneal hemangiopericytomas. Int J Radiat Oncol Biol Phys. 2008;72:1333–1339. doi: 10.1016/j.ijrobp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.H., Jung H.W., Kim Y.S. Meningeal hemangiopericytomas: long-term outcome and biological behaviour. Surg Neurol. 2003;59:47–54. doi: 10.1016/s0090-3019(02)00917-5. [DOI] [PubMed] [Google Scholar]
- 14.Jha N., McNeese M., Barkley H.T., Jr. Does radiotherapy have a role in hemangiopericytoma management? Report of 14 new cases and a review of the literature. Int J Radiat Oncol Biol Phys. 1987;13:1399–1402. doi: 10.1016/0360-3016(87)90236-7. [DOI] [PubMed] [Google Scholar]
- 15.Someya M., Sakata K.I., Oouchi A. Four cases of meningeal hemangiopericytoma treated with surgery and radiotherapy. Jpn J Clin Oncol. 2001;31:548–552. doi: 10.1093/jjco/hye116. [DOI] [PubMed] [Google Scholar]
- 16.Soyuer S., Chang E.L., Selek U., McCutcheon I.E., Maor M.H. Intracranial meningeal hemangiopericytoma: the role of radiotherapy. Report of 29 cases and review of literature. Cancer. 2004;100:1491–1497. doi: 10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.W., Kim D.G., Chung H.T., Paek S.H., Kim Y.H., Han J.H., Park C.K., Kim C.Y., Jung H.W. Gamma knife stereotactic radiosurgery for intracranial hemangiopericytomas. J Neurooncol. 2010;99:115–122. doi: 10.1007/s11060-010-0114-z. [DOI] [PubMed] [Google Scholar]
- 18.Schiariti M., Goetz P., El-Maghraby H., Tailor J., Kitchen N.H. Hemangiopericitoma: long term outcome revisited. Clinical article. J Neurosurg. 2011;114(3):747–755. doi: 10.3171/2010.6.JNS091660. [DOI] [PubMed] [Google Scholar]


