Abstract
Background
Pelvic organs morbidity after irradiation of cancer patients remains a major problem although new technologies have been developed and implemented. A relatively simple and suitable method for routine clinical practice is needed for preliminary assessment of normal tissue intrinsic radiosensitivity. The micronucleus test (MNT) determines the frequency of the radiation induced micronuclei (MN) in peripheral blood lymphocytes, which could serve as an indicator of intrinsic cell radiosensitivity.
Aim
To investigate a possible use of the micronucleus test (MNT) for acute radiation morbidity prediction in gynecological cancer patients.
Materials and methods
Forty gynecological cancer patients received 50 Gy conventional external pelvic irradiation after radical surgery. A four-field “box” technique was applied with 2D planning. The control group included 10 healthy females.
Acute normal tissue reactions were graded according to NCI CTCAE v.3.0. From all reaction scores, the highest score named “summarized clinical radiosensitivity” was selected for a statistical analysis.
MNT was performed before and after in vitro irradiation with 1.5 Gy. The mean radiation induced frequency of micronuclei per 1000 binucleated cells (MN/1000) and lymphocytes containing micronuclei per 1000 binucleated cells (cells with MN/1000) were evaluated for both patients and controls.
An arbitrary cut off value was created to pick up a radiosensitive individual: the mean value of spontaneous frequency of cells with MN/1000 ± 2SD, found in the control group.
Results
Both mean spontaneous frequency of cells with MN/1000 and MN/1000 were registered to be significantly higher in cancer patients compared to the control group (t = 2.46, p = 0.02 and t = 2.51, p = 0.02). No statistical difference was registered when comparing radiation induced MN frequencies between those groups.
Eighty percent (32) of patients developed grade 2 summarized clinical radiosensitivity, with great variations in MNT parameters. Only three patients with grade 2 “summarized clinical radiosensitivity” had values of cells with MN/1000 above the chosen radiosensitivity threshold.
Conclusion
The present study was not able to confirm in vitro MNT applicability for radiosensitivity prediction in pelvic irradiation.
Keywords: Pelvic irradiation, Normal tissue radiosensitivity, Micronucleus test, Predictive methods
1. Background
The contemporary radiotherapy aims at achieving local tumor control with contribution to the overall survival with a good quality of life.
The effects in early responding tissues are a very important issue in radiotherapy.1,2
Normal tissue intrinsic radiosensitivity is the main limiting factor for total irradiation dose in clinical practice defining a normal tissue tolerance to irradiation.3–5
Pelvic organs morbidity after irradiation of cancer patients remains a major problem, although new technologies have been developed and implemented.
A relatively simple and suitable method for routine clinical practice is needed for preliminary assessment of normal tissue intrinsic radiosensitivity. It would allow individualization of radiotherapy dose applying higher dose in radioresistant patients to achieve a potentially better local tumor control of up to 20%.1,6
Multiple studies have shown that normal tissue morbidity after irradiation in cancer patients correlates with radiosensitivity of skin fibroblasts and peripheral blood lymphocytes of these patients. This finding stimulates the search of prognostic criteria for evaluating the intrinsic individual cellular radiosensitivity.1,3,7
The micronucleus test (MNT) determines the frequency of the radiation induced micronuclei (MN) in peripheral blood lymphocytes, which could serve as an indicator of intrinsic cell radiosensitivity.
After mutagen attack, MN in interphase cells are formed by mitotic loss of acentric fragments or chromosomes which are not incorporated in the daughter cell nuclei.8 Because of its reliability and easy performance, MNT could be a promising method for evaluating normal tissue morbidity in cancer patients during radiotherapy1 with results yielded in less than 2 weeks.
The in vitro radiation response of the peripheral blood lymphocytes correlates with the in vivo response.7,9 Human lymphocytes from peripheral blood could be easier to collect with results faster to obtain. Besides, the method is easily reproducible, which makes it a preferred modality in assessing the normal tissue radiosensitivity.1,8,10 Some studies on cancer patients show the association of increased radiosensitivity with side effects.1,10 Study of prostate cancer patients showed association between the frequencies of ex vivo induced MN before the beginning of radiotherapy and gastrointestinal and genitourinary side effects.11 So, there are convincing and logical evidence for using radiation changes in the peripheral blood lymphocytes in the Go phase as a quick prognostic biomarker for normal tissue morbidity.
In the search for the best model for clinical radiosensitivity testing, cervical and endometrial cancers were found to be very appropriate cancer localizations.
2. Aim
In an effort to find a prognostic method for normal tissue intrinsic radiosensitivity for patients receiving pelvic irradiation, we used the micronucleus test (MNT).
We investigated if patients with high frequency of in vitro radiation induced micronuclei in peripheral blood lymphocytes are at high risk for developing more severe early normal tissue adverse events after pelvic irradiation.
3. Materials and methods
3.1. Patients, treatment and control group
The study included 40 females – 23 with cervical cancer and 17 with endometrial cancer in early stage with no lymph node metastasis present. They were recruited for the period of 2006–2008. The mean age was 57.7 ± 13.5 (range 31–75 years). They all received adjuvant external pelvic radiotherapy after radical gynecological surgery with a cobalt machine. The total dose delivered was 50 Gy with 2 Gy daily fractions. A four-field “box” technique was applied. The 2D planning target volume (PTV) inevitably included 2/3 of the vagina, 50% bladder and segments from the rectum and small intestine.
We investigated both cervical and endometrial cancer patients together because the irradiation technique and the PTV we applied were the same according to the treatment protocol of our department for postoperative radiotherapy in early stage for these cancer localizations.
The patients included had no history of previous toxic treatment or exposure.
An informed consent was obtained from every patient before the start of treatment and the study was approved by the local ethic committee.
A control group of 10 healthy females was used. Mean controls’ age was 48.9 ± 12.6 (range 30–66). Our control group was found to be representative when compared with a referent control group of 57 healthy donors of the same Bulgarian laboratory and we stopped recruiting more healthy donors.12
3.2. Normal tissue reactions
Acute normal tissue reactions were graded according to the Common Terminology Criteria for Adverse Events v.3.0 of the NCI. We worked out a radiation reaction questionnaire based on CTCAE v.3.0 for data collecting. The patients were followed weekly during the radiotherapy course, and in the first and third month after it ended for recording side effects defined as early during that period. Gastrointestinal, genitourinary and skin side effects were observed and registered. Besides grading the adverse events from 1 to 5, for statistical evaluation of the data, we created an additional parameter named summarized clinical radiosensitivity. It represented the highest reaction score in any of the systems for an individual patient.
3.3. Micronucleus assay
Two blood samples were obtained prior to the beginning of the radiotherapy from each patient in Li-heparin vaccutainers. After in vitro irradiation of each sample with 1.5 Gy of 60Co source (dose rate 1 Gy/min), the MNT was performed on the lymphocytes. The MN assay began within 24 h after collection of blood samples. Both the irradiated and the control blood was mixed with 5 ml RPMI-1640 culture medium supplemented with 20% calf serum and phytohemagglutinin at 2 mkg/ml final concentration. The samples were incubated for 72 h at 37 °C in 5% CO2. To block the cytokinesis at 44 h cytohalasin B was added to produce final concentration of 6 mkg/l. After total incubation time of 72 h, the lymphocytes were harvested, they were centrifuged and fixed with methanol and acetic acid (3:1) for 15 min. The fixation was done three times and then a cell drop on dry clean slides was spread and stained with 5% Giemsa. MN were scored using the criteria proposed by Fenech.13 For each patient, 2000 binucleated cells were respectively analyzed both on the nonirradiated and irradiated stains.
The total number of MN and lymphocytes with one, two and three MN was defined. The mean frequency of lymphocytes containing MN per 1000 binucleated cells (cells with MN/1000) and the mean frequency of the MN per 1000 binucleated cells (MN/1000) were calculated per sample. Although the two parameters are interrelated and reflect the same process, we analyzed both of them.
For both parameters we calculated: (1) the spontaneous (baseline) frequency, (2) the frequency after in vitro irradiation with 1.5 Gy and (3) the radiation induced frequency.
The radiation induced frequency of cells with MN/1000 and MN/1000 was defined by subtracting the spontaneous frequency from the frequency after irradiation. The same procedure was performed with the healthy donors’ blood samples.
An arbitrary cut off value was created to pick up a radiosensitive individual. It consisted of the mean value of spontaneous frequency of cells with MN/1000 ± 2-fold standard deviation, found in the control group. Such a parameter was proposed by Scott et al.14
3.4. Statistical analysis
Descriptive statistics was used for statistical analysis of the clinical material, radiation adverse events and frequency of cells with MN/1000 and MN/1000. t-Test for independent samples and one-way ANOVA and post hoc Dunnett test were applied for evaluation of differences in the normal tissue radiosensitivity between the groups.
4. Results
4.1. Radiation adverse events
The observed acute side effects in the 40 patients are shown in Table 1. Only 2 patients (5%) did not develop any side effects during the treatment and the follow up period. No severe adverse events of grade 3, 4 and 5 were recorded. The remaining 38 patients had grade 1 and 2 reactions. Gastrointestinal reactions were seen in 38 women (95%), respectively grade 1 in 17.5% and grade 2 in 77.5% which involved the loss of appetite, nausea, vomiting, diarrhea, fecal incontinence, abdominal pains, hemorrhoids, blood and mucus in the stool. Grade 1 genitourinary side effects were reported by 21 patients (52.5%), while 11 patients had grade 2 events (27.5%). The patients complained of dysuria, polyuria, nicturia and incontinence. Grade 1 and 2 skin reactions, including erythema, dry and moist desquamation, were recorded in 11 (27.5%) and 13 (32.5%) patients, respectively.
Table 1.
Early radiation adverse events in 40 gynecological cancer patients.
| Adverse events | Grade according to CTCAE v.3.0 |
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3–5 | |
| Gastrointestinal reactions | 2 (5%) | 7 (17.5%) | 31 (77.5%) | 0 (0%) |
| Genitourinary reactions | 8 (20%) | 21 (52.5%) | 11 (27.5%) | 0 (0%) |
| Skin reactions | 16 (40%) | 11 (27.5%) | 13 (32.5%) | 0 (0%) |
| Summarized clinical radiosensitivity | 2 (5%) | 6 (15%) | 32 (80%) | 0 (0%) |
When different normal tissue reactions in one patient occurred, the highest score from all recorded side effects, named “summarized clinical radiosensitivity” was selected for statistical evaluation of the data.
We concluded that total dose of 50 Gy in the pelvis did not cause severe adverse events – 80% (32) of patients developed grade 2 summarized clinical radiosensitivity after pelvic irradiation of 50 Gy.
4.2. Micronucleus assay
The frequency of cells with MN/1000 and MN/1000 in the control group before irradiation (spontaneous frequency), after irradiation, and the radiation induced frequency are presented in Tables 2 and 3, respectively.
Table 2.
Frequency of cells with MN/1000 in healthy donors group – spontaneous, after irradiation and radiation induced frequency.
| Donor number | Spontaneous frequency of cells with MN/1000 | Frequency of cells with MN/1000 after in vitro irradiation | Radiation induced frequency of cells with MN/1000 |
|---|---|---|---|
| 1 | 8 | 76.5 | 68.5 |
| 2 | 6 | 83.5 | 77.5 |
| 3 | 11 | 84.0 | 73.0 |
| 4 | 23 | 146.0 | 123.0 |
| 5 | 19 | 103.5 | 84.5 |
| 6 | 15 | 117.5 | 103.0 |
| 7 | 20 | 99.0 | 79.0 |
| 8 | 16 | 112.0 | 96.0 |
| 9 | 17 | 104.0 | 87.5 |
| 10 | 23 | 106.5 | 83.5 |
| Mean value ± SD | 15.7 ± 5.9 | 103.25 ± 20 | 87.6 ± 16.1 |
Table 3.
Frequency of MN/1000 in healthy donors group – spontaneous, after irradiation and radiation induced frequency.
| Donor number | Spontaneous frequency of MN/1000 | Frequency of MN/1000 after in vitro irradiation | Radiation induced frequency of MN/1000 |
|---|---|---|---|
| 1 | 8.50 | 83.5 | 75.00 |
| 2 | 7.00 | 92.0 | 85.00 |
| 3 | 11.50 | 94.5 | 83.00 |
| 4 | 26.50 | 162.5 | 136.00 |
| 5 | 20.50 | 113.5 | 93.00 |
| 6 | 16.50 | 138.0 | 121.50 |
| 7 | 23.50 | 112.0 | 88.50 |
| 8 | 19.50 | 134.5 | 115.00 |
| 9 | 16.50 | 117.0 | 100.50 |
| 10 | 25.00 | 125.0 | 100.00 |
| Mean value ± SD | 17.5 ± 6.8 | 117.25 ± 24 | 99.7 ± 19.1 |
The mean radiation induced frequency of cells with MN/1000 in the control group is 87.6 ± 16.1 (Table 2). The mean radiation induced frequency of MN/1000 in the control group is 99.7 ± 19.1 (Table 3). To assess the representativeness of the control group of the study we compared the spontaneous frequency of both parameters with a referent control group of 57 healthy subjects tested in the same laboratory by the same method, with the following reference values: cells with MN/1000 – 11.82 ± 6.25 and MN/1000 12.94 ± 6.99.12 There was no significant difference in the investigated baseline parameters (spontaneous frequency of MN/1000 cells or cells with MN/1000 (t-test for independent samples t = 2.089; p = 0.066; t = 2.122; p = 0.063) between our controls and referent controls.
The frequency of cells with MN/1000 and MN/1000 in the group of 40 patients before irradiation (spontaneous frequency), after irradiation, and the radiation induced frequency are presented respectively in Tables 4 and 5.
Table 4.
Frequency of cells with MN/1000 in patients group – spontaneous, after irradiation and radiation induced frequency.
| Patient number | Spontaneous frequency of cells with MN/1000 | Frequency of cells with MN/1000 after in vitro irradiation | Radiation induced frequency of cells with MN/1000 |
|---|---|---|---|
| 1 | 15 | 138.5 | 123.5 |
| 2 | 10 | 73 | 63 |
| 3 | 23 | 102.5 | 79.5 |
| 4 | 9.5 | 85 | 75.5 |
| 5 | 22 | 87 | 65 |
| 6 | 19 | 77 | 58 |
| 7 | 12.5 | 93 | 80.5 |
| 8 | 19 | 95 | 76 |
| 9 | 15.5 | 104 | 88.5 |
| 10 | 21 | 113 | 92 |
| 11 | 26 | 94 | 68 |
| 12 | 12 | 90.5 | 78.5 |
| 13 | 17 | 99.5 | 82.5 |
| 14 | 52.5 | 160 | 107.5 |
| 15 | 21 | 117.5 | 96.5 |
| 16 | 26.5 | 118 | 91.5 |
| 17 | 4.5 | 88.5 | 84 |
| 18 | 6 | 92.5 | 86.5 |
| 19 | 15.5 | 113 | 97.5 |
| 20 | 17.5 | 91 | 73.5 |
| 21 | 30.5 | 118 | 87.5 |
| 22 | 23.5 | 92 | 68.5 |
| 23 | 17 | 119 | 102 |
| 24 | 19.5 | 73 | 53.5 |
| 25 | 12 | 103.5 | 91.5 |
| 26 | 35 | 168.5 | 133.5 |
| 27 | 35.5 | 114.5 | 79 |
| 28 | 22.5 | 111 | 88.5 |
| 29 | 19.5 | 89.5 | 70 |
| 30 | 34.5 | 102 | 67.5 |
| 31 | 20 | 109 | 89 |
| 32 | 19.5 | 132.5 | 113 |
| 33 | 44.5 | 162.5 | 118 |
| 34 | 38.5 | 146.5 | 108 |
| 35 | 23.5 | 133.5 | 110 |
| 36 | 19 | 140.5 | 121.5 |
| 37 | 15 | 122 | 107 |
| 38 | 16.5 | 132.5 | 116 |
| 39 | 27 | 143 | 116 |
| 40 | 31 | 136 | 105 |
| Mean value ± SD | 21.7 ± 10 | 112 ± 24.6 | 90.3 ± 19.7 |
Table 5.
Frequency of MN/1000 in patients group – spontaneous, after irradiation and radiation induced frequency.
| Patient number | Spontaneous frequency of MN/1000 | Frequency of MN/1000 after in vitro irradiation | Radiation induced frequency of MN/1000 |
|---|---|---|---|
| 1 | 7 | 162.5 | 155.5 |
| 2 | 11 | 80.5 | 69.5 |
| 3 | 26.5 | 125 | 98.5 |
| 4 | 13 | 104.5 | 91.5 |
| 5 | 22.5 | 101 | 78,5 |
| 6 | 20.5 | 88 | 67.5 |
| 7 | 13 | 104 | 91 |
| 8 | 22 | 111 | 89 |
| 9 | 17.5 | 129 | 111.5 |
| 10 | 23.5 | 141 | 117.5 |
| 11 | 31 | 96 | 65 |
| 12 | 13 | 101 | 88 |
| 13 | 18.5 | 112.5 | 94 |
| 14 | 61 | 217.5 | 156.5 |
| 15 | 27 | 137 | 110 |
| 16 | 29 | 134.5 | 105.5 |
| 17 | 4.5 | 102.5 | 98 |
| 18 | 6 | 104 | 98 |
| 19 | 18.5 | 145 | 126.5 |
| 20 | 20 | 109 | 89 |
| 21 | 35 | 131.5 | 96.5 |
| 22 | 26 | 101 | 75 |
| 23 | 25 | 130 | 105 |
| 24 | 20 | 79.5 | 59.5 |
| 25 | 14 | 112 | 98 |
| 26 | 42.5 | 207 | 164.5 |
| 27 | 38 | 132.5 | 94.5 |
| 28 | 26.5 | 123 | 96.5 |
| 29 | 22.5 | 99.5 | 77 |
| 30 | 40.5 | 113 | 72.5 |
| 31 | 23.5 | 127 | 103.5 |
| 32 | 22 | 155.5 | 133.5 |
| 33 | 56 | 194.5 | 138.5 |
| 34 | 42.5 | 175 | 132.5 |
| 35 | 25.5 | 155 | 129.5 |
| 36 | 21 | 162.5 | 141.5 |
| 37 | 17 | 142.5 | 125.5 |
| 38 | 22 | 164.5 | 142.5 |
| 39 | 29 | 173 | 144 |
| 40 | 37 | 162.5 | 125.5 |
| Mean value ± SD | 24.76 ± 12 | 131.16 ± 33.5 | 106.5 ± 27.5 |
The mean spontaneous frequency of cells with MN/1000 and MN/1000 is respectively 21.7 ± 10 and 24.7 ± 12.2. After sample irradiation, the mean frequency of cells with MN/1000 is 112 ± 24.6 and MN/1000 was 131.16 ± 33.5.
Tables 4 and 5 demonstrate the derivation of the two parameters – (1) radiation induced frequency of cells with MN/1000 and (2) radiation induced frequency of MN/1000.
The mean radiation induced frequency of cells with MN/1000 of the 40 patients group is 90.3 ± 19.7.
The mean radiation induced frequency of MN/1000 of the group of 40 patients is 106.5 ± 27.5.
Fig. 1 presents data on spontaneous frequency of cells with MN/1000 and MN/1000 of cancer patient and healthy donor groups.
Fig. 1.
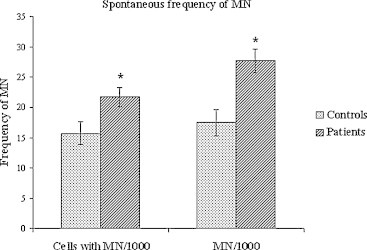
Spontaneous frequency of cells with MN/1000 and MN/1000 of cancer patients and healthy donors groups (mean value ± SEM). *Significant difference between the groups with degree of probability p < 0.05 (t-test).
The mean spontaneous frequency of both parameters in cancer patients (cells with MN/1000 and MN/1000) was significantly higher compared to the control group (t = 2.46, p = 0.021 and t = 2.51, p = 0.018). One-way ANOVA showed a significant effect of the factor diagnosis F (1, 38) = 4.58, p < 0.05) only for spontaneous (non irradiated lymphocytes) frequency MN/1000. Post hoc Dunnett test revealed that the frequency of spontaneous MN/1000 of patients with cervical cancer was statistically reliably lower than that of endometrial carcinoma patients (p < 0.05).
No significant difference was found when comparing radiation induced frequency of cells with MN/1000 and MN/1000 between patient and healthy donor groups. One-way ANOVA did not show a significant effect of the factor diagnosis for the radiation induced frequency of cells with MN/1000 and MN/1000.
4.3. Selection of radiosensitive individuals
The arbitrary cut off value named radiosensitivity threshold is 119.9 cells with MN/1000. It is derived from the mean value of the spontaneous frequency of cells with MN/1000 in the observed control group 87.5 + 2 SD (2 × 16.1). This parameter is considered in the literature as a criterion for high radiosensitivity14 and used by some authors.15 We applied the parameter “cells with MN/1000” which is considered more suitable in evaluating a single cell death risk, while a single MN in diploid cell correlates only with the loss of proliferative activity.16
Fig. 2 shows the values of the radiation induced frequency of cells with MN/1000 in each patient, plotted to summarized clinical radiosensitivity. Only three patients with the highest grade of summarized clinical radiosensitivity in the group (grade 2) had values of cells with MN/1000 above the radiosensitivity threshold, respectively 123.5, 133.5 and 121.5.
Fig. 2.
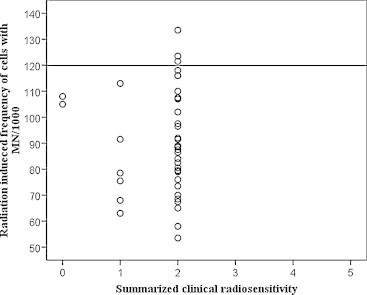
Radiation induced frequency of cells with MN/1000 in cancer patient, compared with summarized clinical radiosensitivity. Straight line shows the limit set by the mean frequency of radiation induced cells with MN/1000 of the control group plus twice the standard deviation (119.9).
Patients with grade 2 side effects showed variable values of MNT on both parameters.
Only one of the three patients with a value of cells with MN/1000 above the radiosensitivity threshold (123.5) had grade 2 genitourinary side effects. The patient with the value of 121.5 cells with MN/1000 (50 years old) had grade 1 reactions, while the third patient with the highest value of 133.5 (70 years old) did not develop genitourinary side effects. The same patient had a low spontaneous frequency of cells with MN/1000 amounting to 35.
Two of the patients with values above the radiosensitivity threshold developed grade 2 skin reactions, and the third patient did not develop any skin reactions (60 years old). To highlight some of the toxic agents, a correlation between induced frequencies of MN in the patient group and smoking was also investigated. No such correlation was found. We did not find smoking to affect the spontaneous cells with MN/1000 (t = 0.68, p = 0.07) and MN/1000 (t = 0.72, p = 0.08).
5. Discussion
MNT is a relatively simple method and its implementation takes several days. This makes it especially attractive and many authors have explored it in order to demonstrate its capabilities for routine clinical use. In most works, the MN assay has been studied more as an indicator of cell liability in development of various malignancies – cancers of the head and neck, breast, lung.10 It is successfully implemented to identify high radiosensitive breast cancer patients.17 These reasons stimulate our efforts to investigate similar association in pelvic irradiation in cancer patients, where the gastrointestinal and genitourinary adverse events significantly deplete patients’ quality of life, compared with cosmetic skin reactions in breast irradiation, assessed in the above mentioned study.
The cohorts of patients in studies reported in the literature are inhomogeneous. They involved patients with different types of malignant tumors, different sizes of PTV and critical organ volumes, different daily and total radiation doses and techniques applied.18,19
Our study group of 40 patients was a homogeneous cohort in terms of gender, clinical stage, previous treatment, equipment, volume, fractionation and total dose of irradiation, which is an advantage of our study compared to other studies. We are the first authors to use the system CTCAE 3.0 when the MNA is applied for radiosensitivity prediction.
Most authors analyzed 100, 250, 500–1000 lymphocytes,14,18–21 especially in cases of low frequency of MN. To increase the accuracy and obtain statistical reliability of the MNT, we examined 2000 cells of patients and controls, both before and after irradiation,22 meeting the recommendations for standardizing the method.23
Once we applied in vitro a test dose of 1.5 Gy aiming to define highly radiosensitive patients prior to the beginning of irradiation, the mean statistical difference between healthy donors and cancer patients disappeared. The same conclusion came from the study of Mozdarani et al., on esophageal cancer patients, who also reported a deletion of MN difference in control and patients groups after in vitro irradiation.15 Some authors, however, found such difference, which may be due to several factors: cancer types, fractionation schedules, total doses applied,11,19 including high-dose brachytherapy.19,20
Studying the frequency of radiation induced MN, we found large individual variations in both the control and patient group. This implies the existence of different individual sensitivity to genotoxic agents, a difference in repair capacity or different patient exposure to chemical genotoxic agents, undetected in the history of controls. To outline some of the agents, we sought correlation between the radiation induced micronucleus frequency in the patient group and smoking. No difference was found in both tested parameters. Probably, that is the reason why Widel et al.20 did not analyze smoking. In addition Ban et al.24 found that smoking did not affect the spontaneous frequency of MN and cells with MN. Moreover, the results of Human MicroNucleus project also showed no increase in MN baseline in smokers.25 Only in long active smokers exposed to occupational genotoxic agents can significant increase in MN be seen. This shows the lack of sensitivity of the MNT to such an important agent in society.
The criterion defining a radiosensitive patient is quite tentative. Fig. 2 shows a mean frequency of radiation induced cells with MN/1000 + two times standard deviation in the control group (95% CI). This parameter was proposed by Scott et al. who also noted that larger groups are needed for more precise definition of a radiosensitivity threshold.14
In our control group, the threshold of radiosensitivity was 119.9 cells with MN/1000. Applying this strongly restrictive criterion, we detected only three patients with values of MN above the threshold witch had developed grade 2 early adverse events (Fig. 2). Such low rate reactions could be explained with moderate dose of 50 Gy to the pelvis organs, routinely applied in clinical practice. We did not include patients on brachytherapy in the study.
Our final analysis points out that MNT did not interpret radiation sensitivity explicitly.
-
1.
After 1.5 Gy in vitro irradiation differences between controls and cancer patients were erased.
-
2.
Large inter-individual variations are observed between patients with grade 2 side effects (ranging from 53.5 to 133 5 cells with MN/1000 and from 59.5 to 164.5 for MN/1000).
-
3.
At the same time, there are patients who did not develop any side effects, but had values of radiation induced MN frequency above the mean.
Therefore, our results could not lead to recommendation of in vitro MNT for routine clinical use. Similar statement could also be found in Maria Widel work, who tested 55 cervical cancer patients, even with higher doses of up to 100 Gy after brachytherapy.20
Doses of 45–50 Gy were routinely applied in clinical practice after radical surgery for cancer of the uterine cervix and endometrium. They were selected after long experience with huge number of patients in many clinical trials. Nevertheless, the application of these doses, tolerated by normal pelvic tissues as they are, could later result in severe radiation proctitis, colitis and cystitis in some individuals and could seriously harm their quality of life. Individual radiosensitivity of such patients was the target of our study aiming to find a reliable test with proper parameters.
6. Conclusion
High expectations were raised over the studies for radiosensitivity prediction in cancer patients aiming at individualization of radiotherapy, prophylaxis and attenuation of adverse events.
Large inter-individual variations in the values of spontaneous and radiation induced frequency of MN were registered in the study. They do not correspond to moderate radiation side effects in the pelvis after application of standard doses of 50 Gy, tolerated by pelvis risk organs. The overlapping frequencies of MN parameters in patients with moderate radiation side effects and patients with none or mild effects were significant.
The final study analysis does not provide evidence supporting direct implementation of in vitro MNT in radiotherapy practice for individual radiosensitivity assessment.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank the team of Radiotherapy Department.
The study was supported by a research grant No. 5-D/2006 at Medical University of Sofia, Bulgaria.
References
- 1.West C.M., Davidson S.E., Elyan S.A. The intrinsic radiosensitivity of normal and tumor cells. Int J Radiat Biol. 1998;73:409–413. doi: 10.1080/095530098142248. [DOI] [PubMed] [Google Scholar]
- 2.Biete A., Valduvieco I., Rovirosa A., Farrus B., Casas F., Conill C. Whole abdominal radiotherapy in ovarian cancer. Rep Prac Oncol Radiother. 2010;15(2):27–30. doi: 10.1016/j.rpor.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnet N.G., Nyman J., Turesson I., Wurm R., Yarnold J.R., Peacock J.H. The relationship between cellular radiation sensitivity and tissue response may provide the basis for individualizing radiotherapy schedules. Radiother Oncol. 1994;33:228–238. doi: 10.1016/0167-8140(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 4.Peacock J., Ashton A., Bliss J. Cellular radiosensitivity and complication risk after curative radiotherapy. Radiother Oncol. 2000;55:173–178. doi: 10.1016/s0167-8140(00)00173-0. [DOI] [PubMed] [Google Scholar]
- 5.West C.M., Davidson S.E., Elyan S.A. Lymphocyte radiosensitivity is a significant prognostic factor for morbidity in carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2001;51:10–15. doi: 10.1016/s0360-3016(01)01575-9. [DOI] [PubMed] [Google Scholar]
- 6.Bentzen S.M. Potential clinical impact of normal-tissue intrinsic radiosensitivity testing. Radiother Oncol. 1997;43:121–131. doi: 10.1016/s0167-8140(97)01899-9. [DOI] [PubMed] [Google Scholar]
- 7.Barber J.B., Burrill W., Spreadborough A.R. Relationship between in vitro chromosomal radiosensitivity of peripheral blood lymphocytes and the expression of normal tissue damage following radiotherapy for breast cancer. Radiother Oncol. 2000;55:179–186. doi: 10.1016/s0167-8140(99)00158-9. [DOI] [PubMed] [Google Scholar]
- 8.Fenech M., Neville S. Conversion of excision-repairable DNA lesions to micronuclei one cell cycle in human lymphocytes. Environ Mol Mutagen. 1992;19:27–36. doi: 10.1002/em.2850190106. [DOI] [PubMed] [Google Scholar]
- 9.Thierens H., Vral A., Van Eijkeren M., Speleman F., De Ridder L. Micronucleus induction in peripheral blood lymphocytes of patients under radiotherapy treatment for cervical cancer or Hodgkin's disease. Int J Radiat Biol. 1995;67:529–539. doi: 10.1080/09553009514550641. [DOI] [PubMed] [Google Scholar]
- 10.Catena C., Conti D., Parasacchi P. Micronuclei in cytokinesis-blocked lymphocytes may predict patient response to radiotherapy. Int J Radiat Biol. 1996;70:301–308. doi: 10.1080/095530096145030. [DOI] [PubMed] [Google Scholar]
- 11.Lee T.K., Allison R.R., O’Brien K.F. Lymphocyte radiosensitivity correlated with pelvic radiotherapy morbidity. Int J Radiat Oncol Biol Phys. 2003;57:222–229. doi: 10.1016/s0360-3016(03)00411-5. [DOI] [PubMed] [Google Scholar]
- 12.Hadjidekova V., Popova L., Hristova R. Chromosome aberrations and micronuclei in peripheral blood lymphocytes of a healthy individuals control group of Bulgarian population. Mod Med. 2007;5–6:20–25. [Google Scholar]
- 13.Fenech M., Morley A.A. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 14.Scott D., Barber J.B., Levine E.L., Burrill W., Roberts S.A. Radiation-induced micronucleus induction in lymphocytes identifies a high frequency of radiosensitive cases among breast cancer patients: a test for predisposition? Br J Cancer. 1998;7(February (4)):614–620. doi: 10.1038/bjc.1998.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozdarani H., Mansouri Z., Haeri S.A. Cytogenetic radiosensitivity of g0-lymphocytes of breast and esophageal cancer patients as determined by micronucleus assay. J Radiat Res (Tokyo) 2005;46(March (1)):111–116. doi: 10.1269/jrr.46.111. [DOI] [PubMed] [Google Scholar]
- 16.Floyd D.N., Cassoni A.M. Intrinsic radiosensitivity of adult and cord blood lymphocytes as determined by the micronucleus assay. Eur J Cancer. 1994;30A:615–620. doi: 10.1016/0959-8049(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 17.Scott D., Barber J.B., Spreadborough A.R., Burrill W., Roberts S.A. Increased chromosomal radiosensitivity in breast cancer patients: a comparison of two assays. Int J Radiat Biol. 1999;75(January (1)):1–10. doi: 10.1080/095530099140744. [DOI] [PubMed] [Google Scholar]
- 18.Rached E., Schindler R., Beer K.T., Vetterli D., Greiner R.H. No predictive value of the micronucleus assay for patients with severe acute reaction of normal tissue after radiotherapy. Eur J Cancer. 1998;34:378–383. doi: 10.1016/s0959-8049(97)00373-0. [DOI] [PubMed] [Google Scholar]
- 19.Slonina D., Klimek M., Szpytma T., Gasinska A. Comparison of the radiosensitivity of normal-tissue cells with normal tissue reactions after radiotherapy. Int J Radiat Biol. 2000;76:1255–1264. doi: 10.1080/09553000050134483. [DOI] [PubMed] [Google Scholar]
- 20.Widel M., Jedrus S., Lukaszczyk B., Raczek-Zwierzycka K., Swierniak A. Radiation-induced micronucleus frequency in peripheral blood lymphocytes is correlated with normal tissue damage in patients with cervical carcinoma undergoing radiotherapy. Radiat Res. 2003;159(June (6)):713–721. doi: 10.1667/0033-7587(2003)159[0713:rmfipb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Djuzenova C.S., Mühl B., Fehn M., Oppitz U., Müller B., Flentje M. Radiosensitivity in breast cancer assessed by the Comet and micronucleus assays. Br J Cancer. 2006;94(April (8)):1194–1203. doi: 10.1038/sj.bjc.6603005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popova L., Hadjidekova V., Hadjieva T., Agova S., Vasilev I. Cytokinesis-block micronucleus test in patients undergoing radioiodine therapy for differentiated thyroid carcinoma. Hell J Nucl Med. 2005;8(January–April (1)):54–57. [PubMed] [Google Scholar]
- 23.Fenech M., Chang W.P., Kirsch-Volders M., Holland N., Bonassi S., Zeiger E. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res. 2003;534(January (1–2)):65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 24.Ban S., Cologne J.B., Fujita S., Awa A.A. Radiosensitivity of atomic bomb survivors as determined with a micronucleus assay. Radiat Res. 1993;134(May (2)):170–178. [PubMed] [Google Scholar]
- 25.Bonassi S., Neri M., Lando C. HUMN collaborative group Effect of smoking habit on the frequency of micronuclei in human lymphocytes: results from the Human MicroNucleus project. Mutat Res. 2003;543(March (2)):155–166. doi: 10.1016/s1383-5742(03)00013-9. [DOI] [PubMed] [Google Scholar]


