Abstract
Purpose
To assess the results of tracheal cancer patients treatment and factors influencing prognosis.
Background
Primary malignant neoplasms of the trachea are rare. The treatment of choice for tracheal carcinomas is resection. Radiation therapy is recommended as a part of radical treatment or for palliation of symptoms.
Materials and methods
Between 1962 and 2006, 50 patients diagnosed with tracheal cancer were treated at the Centre of Oncology in Krakow. The analysis focused on locoregional recurrence-free survival (LRRFS), disease free survival (DFS) and overall survival (OS). Survival rates, univariate and multivariate analyses of prognostic factors were performed using the Kaplan–Meier method, the log rank test and Cox's proportional hazard method, respectively.
For over 40 years, patients were treated using different modalities: surgery followed by radiotherapy (6%), radiotherapy (78%), chemoradiotherapy (8%), and symptomatic treatment (8%).
Results
The 5-year LRRFS was 18%, DFS was 15% and OS was 17%. gender (favoured females) was the only prognostic factor for LRRFS. For OS, the independent prognostic factors were performance status (favoured Karnofsky higher than 80), stage and year of start of the treatment (later than 1988 vs. earlier – 5-year OS 20% vs. 12%).
5-year OS in the following (strongly differentiated over the time) treatment modalities were: surgery followed by radiotherapy (66%), radiotherapy (16%), chemoradiotherapy (0%), and symptomatic treatment (0%).
Of 44 patients treated with radiotherapy symptomatic partial response was observed in 32 patients and follow-up imaging studies revealed complete response in 5 patients, partial response in 25, stable disease in 4 or progressive disease in 4.
Conclusions
Radical treatment in patients in early stage and good performance status seems to be correlated with the improvement of survival. However, despite the fact that results of treatment are poor, radiotherapy offers symptomatic improvement.
Keywords: Tracheal cancer, Radiotherapy, Staging, Prognostic factors
1. Introduction
Malignant tumors of trachea constitute a rare diagnosis accounting for less than 0.1% of all malignancies.1 Since there are no specific respiratory signs and symptoms of the disease, potentially resectable and curable, the diagnosis is considerably delayed until an advanced stage, which a priori deteriorates the prognosis.
The published studies carried out until now allow to indicate some well defined prognostic factors such as histological subtype – adenoid cystic carcinoma (ACC), early stage disease, complete resection and negative surgical airway margins.2,3
Because there are no guidelines based on randomized clinical trials, the choice of treatment modalities and optimal sequence remains an open-ended question. In daily practice, the most frequently applied is surgery with optional post-operative radiotherapy with up to 52% 5-year overall survival in ACC and 39% in SCC.2,4
Radiotherapy plays a significant role as a part of radical combined treatment as well as a palliative approach.5,6
As there are no randomized clinical trials conducted (due to the low incidence of tracheal cancers) so far, we present another retrospective data evaluating prognostic factors and treatment outcome contributing to the knowledge about this neoplasm.
2. Materials and methods
This analysis has been performed in the group of 50 patients with tracheal cancer who were treated at the Centre of Oncology in Krakow between 1962 and 2006. Characteristics of patients are shown in Table 2. Median age was 56 years (range 25–77), 31 patients were male, 19 were female. All patients presented symptoms preceding diagnosis of cancer, most common of them being dyspnoea, cough and hemoptysis. A substantial majority of diagnoses were based on very simple diagnostic tools, e.g. bronchoscopy and chest X-ray, and only the patients treated from the 1990s had additional chest computed tomography. In all the cases diagnosis was confirmed by histology examination and the most common histological type was squamous cell cancer observed in 48%. The distribution of all histological types is shown in Table 2.
Table 2.
Characteristics of patients.
| Characteristics | n | % |
|---|---|---|
| Age | ||
| Median | 56 | |
| Range | 25–77 | |
| Sex | ||
| Female | 19 | 38 |
| Male | 31 | 62 |
| Performance status (Karnofsky) | ||
| <80 | 35 | 70 |
| ≥80 | 15 | 30 |
| Histological subtype | ||
| Squamous cell carcinoma | 24 | 48 |
| Adenoid cystic carcinoma | 5 | 10 |
| Adenocarcinoma | 6 | 12 |
| Nondifferentiated | 2 | 4 |
| Small cell carcinoma | 4 | 8 |
| Ca solidum | 3 | 6 |
| Others, unknown | 6 | 12 |
| Stage | ||
| I | 5 | 10 |
| II | 21 | 42 |
| IIIA | 18 | 36 |
| IIIB | 5 | 10 |
| IV | 1 | 2 |
| Type of treatment | ||
| Surgery followed by radiotherapy | 3 | 6 |
| Radiotherapy alone | 39 | 78 |
| Chemoradiotherapy | 4 | 8 |
| Symptomatic treatment | 4 | 8 |
| Radiotherapy | ||
| Without radiotherapy | 4 | 8 |
| Pallative | 20 | 40 |
| Radical | 26 | 52 |
| Year of start of treatment | ||
| 1962–1988 | 22 | 44 |
| 1989–2006 | 28 | 56 |
Patients were treated using different treatment modalities: surgery followed by radiotherapy (6%), radiotherapy alone (78%), chemoradiotherapy (8%), and symptomatic treatment (8%).
Of 46 irradiated patients, 26 were treated with radical and 20 with palliative intent. Median external beam radiotherapy dose was 6400 cGy (range 5600–7000) and 3500 cGy (range 2000–4000), in radical and palliative treatment respectively, while in brachytherapy cases it was 15 Gy in two fractions. In radical treatment, dose per fraction ranged from 180 to 200 cGy, while in palliative treatment it ranged from 300 to 400 cGy.
Radiotherapy was delivered by linear accelerator (24 pts.), cobalt (20 pts.) and HDR brachytherapy (2 pts.).
Radiotherapy techniques changed during the period of this analysis.
In the radical treatment two antero-posterior or three oblique fields techniques were used in the first phase, with limitation of the dose to the spinal cord to 45 Gy, using two or three oblique fields in the second phase. Irradiated volume in the first phase included trachea with tumor and enlarged lymph nodes, upper mediastinal lymph and/or cervical nodes electively, in second phase tumor or tumor bed with margins only. Until 1995 two-dimensional (2D) treatment planning was used. From 1996 computed tomography was incorporated into the planning system at the Centre of Oncology in Krakow.
Palliative treatment included tumor and enlarged lymph nodes with margin, mostly in 2D treatment planning setting.
Brachytherapy dose was specified at 1 cm from the axis of the catheter.
All four patients in combined modality group (chemoradiotherapy) received etoposid and cisplatin after radiotherapy. Before the year 2000 systemic treatment was used in distant failure cases only.
Since there is no international tracheal cancer staging system, for the purpose of this analysis we proposed the system shown in Table 1, based on earlier reports from the Centre of Oncology in Krakow.3 About 90% of patients had tumor limited to chest.
Table 1.
Proposal of staging system.
| Stage |
|---|
| I – limited to trachea |
| II – limited to chest |
| IIIA – limited to chest + mediastinal lymph nodes involvement |
| IIIB – limited to chest + supraclavicular lymph nodes involvement |
| IV – metastatic disease |
Majority (66%) of tumors were located in the lower third of the trachea and on the lateral (26%) and posterior wall (18%).
2.1. The statistical methods
The main endpoints of the analysis were locoregional recurrence-free survival (LRRFS), disease free survival (DFS) and overall survival (OS) rates.
Locoregional recurrence-free survival was measured from the date of treatment to the date of locoregional recurrence or death or last follow-up only in the group of patients who responded completely to the treatment or radical resection. Local failure was defined as a failure occurring inside the mediastinum and supraclavicular region (and/or with invasive growth into neighbouring organs) and distant failure as any site of failure outside the mediastinum and supraclavicular region.
Survival was measured from resection to the date of death or lost to follow-up.
Time to progression was measured from the date of the end of treatment to the date of local or distant progression.
Comparison included several clinical (sex, age, performance status, stage, grade, histological type, location of tumor, etc.) and treatment-related factors (type of treatment, intention of radiotherapy, radiation dose, year of start of treatment). Survival rates were calculated using the Kaplan–Meier method. Univariate and multivariate analyses of prognostic factors were performed using the log rank and Cox's proportional hazard methods, respectively. P-values of less than 0.05 were considered to indicate statistical significance.
3. Results
3.1. Survival
During a median follow-up of 9 months (range 1–136), we observed 47 cancer deaths. Only 3 patients were alive in October 2008 of whom 2 were disease free.
The 5-year LRRFS rate, DFS rate and OS rate were 18%, 15% and 17%, respectively. Median survival for all patients was 8.7 months (range 1–139).
The analysis (Tables 3 and 4) showed that only gender had significant impact on LRRFS. The risk of locoregional recurrence in females was less than one-third of the risk in males (29 vs. 9% 5-year LRRFS).
Table 3.
Univariate analysis of prognostic factors (significant bolded).
| Prognostic factors | 5-Year LRFS% | 5-Year DFS% | 5-Year OS% |
|---|---|---|---|
| Total group | 18 | 15 | 17 |
| Sex | P = 0.02 | P = 0.04 | |
| Female | 29 | 24 | 32 |
| Male | 9 | 9 | 7 |
| Age | |||
| >56 year | 31 | 22 | 8 |
| ≤56 year | 9 | 9 | 26 |
| Histological subtype | P = 0.04 | ||
| Ca planoepitheliale | 11 | 11 | 9 |
| Adenoid cystic carcinoma | 33 | 33 | 80 |
| Adenocarcinoma | 0 | 0 | 17 |
| Nondifferentiated | NA | NA | 0 |
| Small cell carcinoma | 0 | 0 | 0 |
| Ca solidum | NA | 50 | 33 |
| Others, unknown | 0 | 0 | 0 |
| Localisation | |||
| 1/3 upper | 0 | 0 | 12.5 |
| 1/3 middle | 0 | 0 | 29 |
| 1/3 lower | 24 | 24 | 16 |
| Not applicable | NA | NA | 0 |
| Localisation | |||
| Anterior wall | 25 | 25 | 14 |
| Posterior wall | 0 | 0 | 22 |
| Lateral wall | 25 | 25 | 9 |
| Circumferential | 0 | 0 | 0 |
| Not applicable | 19 | 17 | 19 |
| Year of start of treatment | P = 0.04 | ||
| 1962–1988 | 13 | 11 | 12 |
| 1989–2006 | 23 | 18 | 20 |
| Type of treatment | P = 0.005 | ||
| Surgery followed by radiotherapy | 0 | 0 | 66 |
| Radiotherapy alone | 21 | 18 | 16 |
| Chemoradiotherapy | NA | NA | 0 |
| Symptomatic treatment | NA | NA | 0 |
| Stage | P = 0.04 | ||
| I | NA | NA | 60 |
| II | 10 | 9 | 14 |
| IIIA | 29 | 14 | 12 |
| IIIB | NA | NA | 0 |
| IV | NA | NA | 0 |
| Radiotherapy | P = 0.01 | ||
| Without radiotherapy | 0 | 0 | 0 |
| Pallative | 0 | 0 | 0 |
| Radical | 21 | 18 | 31 |
| Performance status (Karnofsky) | P = 0.002 | ||
| <80 | 19 | 9 | 3 |
| ≥80 | 21 | 21 | 47 |
NA – not applicable.
Table 4.
5 years multivariate analysis of prognostic factors.
| Variable | Relative risk | P-value | |
|---|---|---|---|
| LRFS | Gender | 0.32 | 0.028 |
| OS | Stage | 1.5 | 0.02 |
| Performance status | 0.19 | 0.00006 | |
| Year of start of treatment | 0.24 | 0.0001 | |
There were no prognostic factors influencing DFS.
In the univariate analysis, histological subtype, gender, stage, type of treatment, year of start of treatment, performance status, and intention of radiotherapy delivery were all statistically significant prognostic factors for OS.
The 5-year overall survival of patients with adenoid cystic carcinoma (80%) was significantly better than that for all other histological types (P < 0.002). Two of 5 cases of adenoid cystic carcinoma underwent surgery, which was combined with radiotherapy, and three underwent radical radiotherapy. Five-year overall survival of most common subtype – squamous cell cancer – was 9%.
Females had longer overall survival than males (32 vs. 7%) so had patients who started treatment after 1988 vs. those treated before 1988 (20% vs. 12%).
5-Year OSs in the following treatment modalities were: 66% for surgery followed by radiotherapy, 16% for radiotherapy alone, 0% for chemoradiotherapy, and 0% for symptomatic treatment.
For OS (Table 4), the independent prognostic factors were stage, performance status and year of start of the treatment. The risk of death was more than one and a half in advanced stage patients (Fig. 1). Patients with higher than 80 Karnofsky performance status and treated after 1988 had less than one-third risk of death compared with patients in poorer performance status and treated before 1988.
Fig. 1.
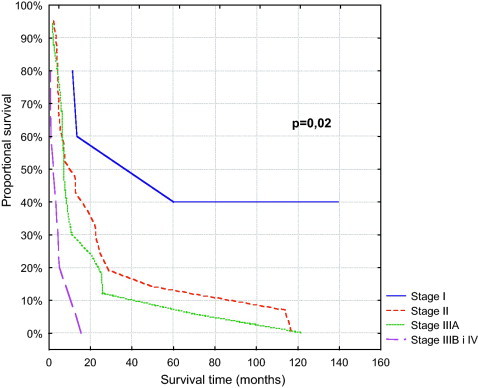
Overall survival.
Of 38 evaluated patients treated with radiotherapy follow-up imaging studies (X-ray, CT or bronchoscopy), complete response occurred in 5 patients, partial response in 25, stable disease in 4 and progressive disease in 4 (in 8 patients imaging studies were not performed).
Symptomatic response assessment was performed in 38 radiotherapy patients. 32 patients (73%) had partial response, 2 had stable disease and 4 progressed.
3.2. Analysis of failure
During the follow-up, local recurrence of malignancy was observed in 19 patients (73% of 26 evaluable patients). Local recurrence was located inside in 5 patients and outside in 5 patients, in 9 cases data could not been obtained. In 12 patients (46%) distant failures were observed, all of them were chemonaive. In 8 cases distant failures were located in the lungs; in 3 it was found in the bones, in 2 in the liver and brain. Median time to both local and distant recurrences was 9 months.
4. Discussion
Evidence in tracheal cancer treatment is based mostly on small retrospective series (usually less than 300), which enrolled patients during long (two, three decades) periods during which treatment and diagnostic modalities were changing.7,8 This is also a limitation of the present study.
Clinical characteristics of our series of patients are similar to that reported in literature. Majority of patients were males and most patients were in the sixth and seventh decade of life.3,4,9–11 Most common symptoms were dyspnoea, cough and haemoptysis, which is in accordance with the largest series4 studied, but in many other, it is haemoptysis that is reported as the most common.6,9 Squamous cell cancer was the most common histological type followed by adenoid cystic carcinoma.10,12–14
Only 3 (6%) patients were operated, and the majority of them were irradiated only, which can be ascribed to selection bias. The majority of tracheal cancer patients in our region had undergone resections in other hospitals, and frequently they had not been referred to radiotherapy. It was shown in an epidemiological study by Honings that tracheal tumors are commonly misclassified and undertreated.15
Therefore results of treatment are inferior to national registers and similar to reported single radiotherapy series and to unresectable subgroup.1,6,13,16
Because of the lack of an international tracheal cancer staging system, we designed one, only for the purpose of this analysis, and the univariate and multivariate analyses of OS found it a significant prognostic factor. This staging system is biased as the period analysed in the study was long and diagnostic modalities changed in the meantime. AJCC criteria for lung cancer stage all tracheal tumors as stage IV. There is another staging system proposed by Bhattacharyya which does not take into consideration neither distant metastases nor location of the nodal involvement.17 Nevertheless, this staging system also predicts survival. The comparison between our staging system and that proposed by Bhattacharyya is shown in Table 5.
Table 5.
Comparison of staging system designed by authors and designed by Bhattacharyya.17
| Designed by authors | Bhattacharyya proposal | ||
|---|---|---|---|
| Stage | 5-Year OS | 5-Year OS | |
| I – limited to trachea | 60% | I – primary tumor confined to trachea, size <2 cm, no evidence of regional nodal disease | 52.8% |
| II – primary tumor confined to trachea, size >2 cm, no evidence of regional nodal disease | 70% | ||
| II – limited to chest | 14% | III – spread outside the trachea but not adjacent organs of structures, no evidence of regional nodal disease | 75% |
| IV – spread to adjacent organs of structures, no evidence of regional nodal disease | 15% | ||
| IIIA – limited to chest + mediastinal lymph nodes involvement | 12% | IV – evidence of regional nodal disease | |
| IIIB – limited to chest + supraclavicular lymph nodes involvement | 0% | ||
| IV – metastatic disease | 0% | None |
The prognosis for adenoid cystic carcinomas is significantly better than that for squamous cell tracheal cancers in the univariate analysis, which supports results of other studies.4,13,18
We found also that resection of tracheal cancer followed by radiotherapy was associated with improved survival.4,16,19
Patients who started to be treatment after 1989 had better overall survival than those who were treated earlier which can be explained by improvement of imaging and radiation delivery methods (the use of modern radiotherapy techniques and higher energies).
The risk of locoregional recurrence in females was less than one-third of the risk in males. This is not in agreement with other papers,5 but patients with squamous cell tracheal cancers (who have worse prognosis) were more likely to be male.4
In our series local relapses are one and half times more common than distant ones, this ratio is similar to that reported by Gaissert, but the proportion of relapsed patients to total tracheal patients is much higher.4 This can be explained by a higher number of patients with advanced disease compared to other series.4
In 38 evaluable patients treated with radiotherapy, response rate assessed by follow-up imaging studies was 73%, whereas in other studies it was heterogeneous ranging between 26% and 73%.5,6,9,20
There were only two side effects of radiotherapy: pneumonitis and oesophagitis (both grade 2). The frequency and severity of side effects of radiotherapy were very low because of underreported side effects in retrospective studies.
Results of this study should be interpreted with caution because of a small number of patients, retrospective design, and time period of the study which are all important limitations of the study.
5. Conclusions
Radical treatment in patients in early stage and good performance status are correlated with improvement of survival.
Despite the fact that results of treatment are pessimistic, radiotherapy offers symptomatic improvement.
References
- 1.Macchiarini P. Primary tracheal tumours. Lancet Oncol. 2006;7(1):83–91. doi: 10.1016/S1470-2045(05)70541-6. [DOI] [PubMed] [Google Scholar]
- 2.Bittner N., Koh W.J., Laramore G.E., Patel S., Mulligan M.S., Douglas J.G. Treatment of locally advanced adenoid cystic carcinoma of the trachea with neutron radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(2):410–414. doi: 10.1016/j.ijrobp.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Rosset A., Korzeniowski S. Effectiveness of radiotherapy in patients with cancer of the trachea. Nowotwory. 1990;40(3):207–213. [PubMed] [Google Scholar]
- 4.Gaissert H.A., Grillo H.C., Shadmehr M.B., Wright C.D., Gokhale M., Wain J.C. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. 2004;78(6):1889–1896. doi: 10.1016/j.athoracsur.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 5.Skowronek J., Adamska K., Zwierzchowski G., Cofta S.Z., Świrkocki K., Piorunek T. HDR brachytherapy in palliative treatment of tracheal carcinoma. Współcz Onkol. 2001;5(1):34–37. [Google Scholar]
- 6.Makarewicz R., Mross M. Radiation therapy alone in the treatment of tumours of the trachea. Lung Cancer. 1998;20(3):169–174. doi: 10.1016/s0169-5002(98)00018-x. [DOI] [PubMed] [Google Scholar]
- 7.Puri T., Gunabushanam G., Malik M., Kukreja M., Julka P.K., Rath G.K. Long-term clinical remission following radiotherapy in tracheal adenocarcinoma: review of the published work. Australas Radiol. 2007;51(5):485–488. doi: 10.1111/j.1440-1673.2007.01703.x. [DOI] [PubMed] [Google Scholar]
- 8.Suemitsu R., Okamoto T., Maruyama R., Wataya H., Seto T., Ichinose Y. A long-term survivor after aggressive treatment for tracheal adenoid cystic carcinoma: a case report. Ann Thorac Cardiovasc Surg. 2007;13(5):335–337. [PubMed] [Google Scholar]
- 9.Jeremic B., Shibamoto Y., Acimovic L., Milisavljevic S. Radiotherapy for primary squamous cell carcinoma of the trachea. Radiother Oncol. 1996;41(2):135–138. doi: 10.1016/s0167-8140(96)01797-5. [DOI] [PubMed] [Google Scholar]
- 10.Czajkowski W., Olechnowicz H., Gawrychowski J. Follow-up study of the treatment of patients for tracheal tumours. Kardioch Torakochirurgia Pol. 2007;4(2) [Google Scholar]
- 11.Thotathil Z.S., Agarwal J.P., Shrivastava S.K., Dinshaw K.A. Primary malignant tumors of the trachea – the Tata Memorial Hospital experience. Med Princ Pract. 2004;13(2):69–73. doi: 10.1159/000075631. [DOI] [PubMed] [Google Scholar]
- 12.Harms W., Latz D., Becker H., Gagel B., Herth F., Wannenmacher M. Treatment of primary tracheal carcinoma. The role of external and endoluminal radiotherapy. Strahlenther Onkol. 2000;176(1):22–27. doi: 10.1007/pl00002300. [DOI] [PubMed] [Google Scholar]
- 13.Regnard J.F., Fourquier P., Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg. 1996;111(4):808–813. doi: 10.1016/s0022-5223(96)70341-0. [DOI] [PubMed] [Google Scholar]
- 14.Hazama K., Miyoshi S., Akashi A., Yasumitsu T., Maeda H., Nakamura K., Matsuda H. Clinicopathological investigation of 20 cases of primary tracheal cancer. Eur J Cardiothorac Surg. 2003;23:1–5. doi: 10.1016/s1010-7940(02)00728-5. for the Thoracic Surgery Study Group of Osaka University. [DOI] [PubMed] [Google Scholar]
- 15.Honings J., Gaissert H.A., Verhagen A.F., van Dijck J.A., van der Heijden H.F., van Die L., Kaanders J.H. Undertreatment of tracheal carcinoma: multidisciplinary audit of epidemiologic data. Ann Surg Oncol. 2009;16(2):246–253. doi: 10.1245/s10434-008-0241-3. [DOI] [PubMed] [Google Scholar]
- 16.Honings J., van Dijck J.A., Verhagen A.F., van der Heijden H.F., Marres H.A. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol. 2007;14(2):968–976. doi: 10.1245/s10434-006-9229-z. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg. 2004;131(5):639–642. doi: 10.1016/j.otohns.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Grillo H.C., Mathisen D.J. Primary tracheal tumors: treatment and results. Ann Thorac Surg. 1990;49(1):69–77. doi: 10.1016/0003-4975(90)90358-d. [DOI] [PubMed] [Google Scholar]
- 19.Webb B.D., Walsh G.L., Roberts D.B., Sturgis E.M. Primary tracheal malignant neoplasms: the University of Texas MD Anderson Cancer Center experience. J Am Coll Surg. 2006;202(2):237–246. doi: 10.1016/j.jamcollsurg.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Cheung A.Y. Radiotherapy for primary carcinoma of the trachea. Radiother Oncol. 1989;14(4):279–285. doi: 10.1016/0167-8140(89)90139-4. [DOI] [PubMed] [Google Scholar]


