Abstract
Aim
The aim of our study was the dosimetric and physical evaluation of the CK and IMRT treatment plans for 16 patients with localized prostate cancer.
Background
Intensity modulated radiation therapy (IMRT) is one of the recent technical advances in radiotherapy. The prostate is a well suited site to be treated with IMRT. The challenge of accurately delivering the IMRT needs to be supported by new advances such as image-guidance and four-dimensional computed conformal radiation therapy (4DCRT) tomography. CyberKnife (CK) provides real time orthogonal X-ray imaging of the patient during treatment course to follow gold fiducials installed into the prostate and to achieve motion correlation between online acquired X-ray imaging and digital reconstructed radiographs (DRRs) which are obtained from planning computed tomography images by translating and rotating the treatment table in five directions.
Methods and materials
Sixteen IMRT and CK plans were performed to be compared in terms of conformity (CI), heterogeneity indices (HI), percentage doses of 100% (V100), 66% (V66), 50% (V50), 33% (V33) and 10% (V10) volumes of the bladder and rectum. Dose-volume histograms for target and critical organs, (CI) and indices (HI) and isodose lines were analyzed to evaluate the treatment plans.
Results
Statistically significant differences in the percentage rectal doses delivered to V10, V33, and V50 of the rectum were detected in favor of the CK plans (p values; <0.001, <0.001 and 0.019, respectively). The percentage doses for V66 and V100 of the rectum were larger in CK plans (13%, 2% in IMRT and 21%, 3% in CK plans, respectively). Percentage bladder doses for V10 and V33 were significantly lower in CK plans [96% in IMRT vs 48% in CK (p < 0.001) and 34% in IMRT vs 24% in CK (p = 0.047)]. Lower percentage doses were observed for V50, V66 of the bladder for the IMRT. They were 5.4% and 3.45% for IMRT and 13.4% and 8.05% for CK, respectively. Median CI of planning target volume (PTV) for IMRT and CK plans were 0.94 and 1.23, respectively (p < 0.001).
Conclusion
Both systems have a very good ability to create highly conformal volumetric dose distributions. Median HI of PTV for IMRT and CK plans were 1.08 and 1.33, respectively (p < 0.001).
Keywords: Prostate, IMRT, CyberKnife, Radiosurgery
1. Introduction
Intensity modulated radiation therapy (IMRT) is one of the recent technical advances in radiotherapy. IMRT is an extension of three-dimensional radiation therapy (3DCRT) that uses all the components of 3DCRT such as dose-volume histograms (DVHs) and target-normal tissue prescriptions. IMRT provides better conformal dose shape especially in cases with concave tumors using intensity modulated fields. Many studies have reported that IMRT was superior to 3DCRT especially in head and neck, prostate and cervical cancer cases.1–6
The prostate is a well suited treatment site to use IMRT. IMRT has been proved to be an effective treatment technique for prostate cancer due to its improved target dose while avoiding doses to the bladder and rectum. Compared to 3DCRT, IMRT significantly reduces late and acute toxicities of radiotherapy and allows prostate dose escalation.7–9 Zelefsky et al.10 published the results of 772 prostate patients who were treated with IMRT and reported that IMRT increased the tumor control probability while decreasing complication rates in normal structures.
Although IMRT represents better tumor control probability, because of its complexity, it needs further quality assurance program to ensure coincidence of planned and the delivered dose.11 Patient set-up errors, systematic errors and organ motions during a treatment course may also reduce the success of IMRT. Organ movements may lead to displacement of the target to indented low isodose lines while organs at risk may be in the high dose region. Numerous reports have been published showing that prostate movements and clinical uncertainties for prostate IMRT must be realistically assessed by the margins of planning target volume (PTV) and displacements of the fiducials inserted to the prostate with daily portal imaging.12–14 The challenge of accurately delivering IMRT needs to be supported by facilities such as Image-Guided Radiotherapy (IGRT), four-dimensional conformal radiation therapy (4DCRT) or CyberKnife (CK) robotic radiotherapy.
Aubin et al.15 have shown that gold marker seeds can be placed into the prostate for routine daily imaging by Electronic Portal Imaging (EPID) device and can increase accuracy of treatment. Although daily ultrasound-based imaging to define prostate localization can improve the precision of IMRT for prostate cancer cases, application of the technique needs physician participation in each treatment.
However, CK provides real time orthogonal X-ray imaging of the patient during a treatment course to follow gold fiducials inserted into the prostate and movement correlation comparing on-line acquired X-ray imaging and digital reconstructed radiographs (DRRs) which are obtained from planning computed tomography (CT) images by translating and rotating the treatment table in five directions. These capabilities of CK enable us to treat prostate cancer with less than 1 mm accuracy. It therefore can allow us to reduce the margins of target so that the incidental doses at the bladder and rectum are minimized.
CK is a frameless whole-body image guided robotic radiosurgery system that has a 6MV linear accelerator mounted on a computer controlled robotic arm and an orthogonal pair of diagnostic X-ray imaging devices (see Fig. 1). CK can irradiate the target using 1200 points in the room. It has the advantage of delivering more doses to the target while avoiding doses to normal structures.
Fig. 1.
CK robotic radiosurgery system.
In addition to dose escalation, hypo-fractionation might also increase the therapeutic ratio due to low α/β of prostate tumor cells. There have been many studies to investigate hypo-fractionation for prostate cancer showing that large doses per fraction allow to achieve the same or even better tumor control while maintaining the same level of normal tissue late effects and acute toxicity.16,17
Clinical policy in our center for localized prostate cancer treatment with CK is to administer 36.25 Gy in five fractions with a mean duration of session of 1.5–2 h. When IMRT is used, patients are treated to a total dose of 72–74 Gy with 2 Gy per fraction in 35–37 fractions.
The aim of our current study is to evaluate dosimetric and physical aspects of CK and IMRT treatment plans for localized prostate cancer. DVHs for target and critical organs, conformity index (CI), heterogeneity index (HI) and isodose lines were employed to evaluate the treatment plans with retrospectively prepared IMRT and CK plans for sixteen patients with localized prostate cancer.
2. Materials and methods
2.1. Patients and imaging
Sixteen patients with localized prostate cancer staged T1–T2 according to the TNM classification 2002 (TNM Classification of Malignant Tumors) were included in our study.18 Median age of the patients was 72. Thirteen of the selected patients were treated with IMRT and other 3 patients were treated with CK in our center. According to our clinical policy, as explained in the section IMRT and CK plans below, IMRT and CK plans were prepared for sixteen patients who were treated in our clinic with CK or IMRT.
Patients were immobilized using an alpha cradle at a supine position with full bladder for IMRT. CT scans from the fifth lumbar vertebrae level to 10 cm below the ischial tuberosities in 3 mm slice thickness were performed on a flat couch. After CT scan, magnetic resonance (MR) scanning was performed to acquire T2-weighted imaging in 3 mm slice thickness to delineate the prostate and regions of interest (ROI) accurately. CT and MR images were transferred to a virtual simulation program to delineate regions of interest. Our virtual simulation program (FocalSim, Computed Medical System, St. Louis) is capable of fusing CT and MR images by Mutual Information (MI).
Experienced radiation oncologist delineated the prostate as a target and the bladder, rectum, femoral heads as critical structures using CT and MR fusion. The following ROIs were defined: Gross Tumor Volume (GTV) consisted of the prostate without margin; Clinical Target Volume (CTV) included the primary GTV with 1 cm margin in all directions except posterior direction. A margin of 0.5 cm was given from posterior direction for CTV to reduce the rectum wall dose. In terms of set-up errors and beam penumbra, PTV was created with 0.5 cm for every direction and beams were conformed to PTV without margin. Delineated CT images for all patients were transferred to an inverse planning system (XiO, Computed Medical System, St. Louis) to generate IMRT plan. The delineated structures for IMRT and CK plans can be seen in Fig. 2(a) and (b).
Fig. 2.
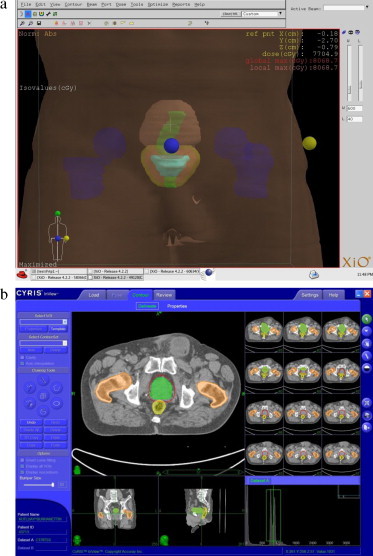
(a) Delineated ROI’ IMRT plan; bladder (brown), cyan (GTV), CTV (red), PTV (yellow), rectum (green), femoral heads (blue). (b) Delineated ROI’ CyberKnife. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.
Although CK has its own imaging protocol for the treatment of localized prostate cancer,19 CT and MR data sets, which were obtained for IMRT, were used to delineate structures for the CK inverse planning system to obtain same volumes of ROIs. CK treatment includes real time image guidance for all treatment sites such as skull tracking for head, implanted fiducial tracking for prostate and spine cases. At least a week before the treatment, gold seeds are implanted to prostate by radiologists, 1 mm slice thickness CT and MR images are acquired in a supine position with alpha cradle. Generally, a foley catheter is placed and the balloon filled with contrast medium to outline the urethra.
A experienced radiation oncologist, who delineated the structures for IMRT plans, delineated ROIs on fused CT and MR images on the CK contouring computer (Cyris Inview, Accuray, Sunnyvale, CA). CK allows delivery and tracking with a precision of less than 1 mm error. The target coverage within the prescribed isodose at CK plan was within 3–5 mm from the contoured target. After definition of GTVs without margin to the prostate, PTVs were created automatically with 0.5 cm margin to GTV in all directions.
2.2. IMRT and CK plans
IMRT plans for 16 patients were generated using the CMS XiO planning system for a 6 MV linear accelerator (Siemens Medical Solution, Conrad) with 41 pairs Multileaf Collimator (MLC) using the step and shoot technique. All IMRT plans, with one exception, were developed using a five-field arrangement including anterior (0°), left anterior oblique (72°), left posterior oblique (144°), right posterior oblique (216°) and right anterior oblique (288°) fields. In only one patient, plan was done by placing the beams starting from the posterior of the patient (180°) due to the patient's anatomy. Fig. 3 shows the beam orientation of a sample IMRT plan. The beams were placed at the center of the PTV and conformed PTV to minimize penumbra effect around the PTV. Inverse planning using a convolution calculation algorithm was used to minimize a physical objective of the function between the calculation and prescribed dose values in the target volume, as well as tolerance dose values for critical structures.
Fig. 3.
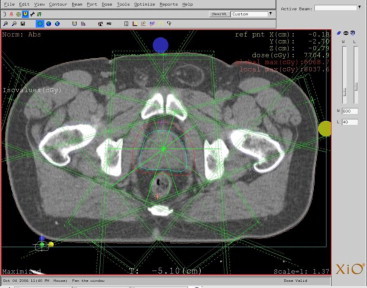
Beam placement of IMRT plan on the XIO.
Pollack et al.20 and Zelefsky et al.21 published articles including large numbers of patients and showing that IMRT treatment with >74 Gy in patients with PSA > 10 ng/mL significantly improved the freedom from failure (FFF) time while decreasing the rectal reactions. Our protocol was designed to reach a total dose of 74 Gy to the PTV in 37 fractions with 2 Gy per fraction. The dose distribution was gained according to the prescription of ROIs as shown in Table 1.
Table 1.
IMRT plan dose prescription of PTV, bladder, rectum and femoral heads.
| Structure | Type | Rank | Objective | Dose (cGy) | Volume (%) | Weight | Power |
|---|---|---|---|---|---|---|---|
| PTV | Target | 1 | Max | 7550 | 0 | 100 | 2.4 |
| Min | 7474 | 100 | 200 | 2.9 | |||
| Bladder | OAR | 2 | Dose volume | 5000 | 25 | 100 | 2 |
| Dose volume | 3100 | 50 | 100 | ||||
| Rectum | OAR | 3 | Dose volume | 5000 | 17 | 100 | 2 |
| Dose volume | 3100 | 35 | 100 | ||||
| Right femoral heads | OAR | 4 | Max | 5000 | 0 | 100 | 2 |
| Left femoral heads | OAR | 4 | Max | 5000 | 0 | 100 | 2 |
| Patient | OAR | 5 | Max | 4500 | 0 | 100 | 2 |
Plan acceptability was considered by comparing dose volume values with defined dose values for PTV and OARs. Table 2 shows an accepted IMRT plan that maximum dose of femoral heads under 50 Gy, maximum dose of plan under 110% of prescribed dose. Our planning system can generate segments using discretization of the ideal intensity map. We limited the number of discrete levels of segmentation to obtain a reasonable subfield number; otherwise it leads to longer treatment times. Segment numbers of plans were generally kept under 70 to keep the treatment time per fraction at about 18 min. The segment number of an accepted plan was 67 as shown in Table 2. Because of the step and shoot technique, the treatment time covers “beam on” time and MLC movements between segments.22,23
Table 2.
An accepted IMRT plan index PTV and OAR's according to defined criteria.
During evaluation of the plans, the dose distributions were also investigated to ensure that there were no low dose region within the CTV and no hotspots in uncontoured tissues. Our radiation oncologist judged the plan according to clinical policy in some plans in which ideal dose distribution could not be achieved because of the energy or patient anatomy. Consequently, our plan evaluation criteria were the following:
-
(1)
95% of PTV must receive 74 Gy and no more than 5% of PTV receives 105% of 74 Gy.
-
(2)
No more than 10% of the rectum and bladder volumes receive more than 70 Gy.
-
(3)
Maximum dose for femoral heads is 50 Gy.
-
(4)
The global maximum dose value of the plan must be less than 110% of 74 Gy.
-
(5)
The hotspots of the plan must not overlap regions of the rectum and bladder with PTV.
It was not always easy to achieve results such as bladder and rectum doses fitting to above mentioned criteria. Therefore, we considered tolerance of the patient and followed the toxicity of the treatment. In 8 plans, the bladder and rectum volumes receiving >70 Gy were larger than 10% of the volume.
CK plans were generated using the On-Target (Accuray, Sunnyvale) planning system which uses a non-optimized solution with a linear programming algorithm. Table 3 shows prescription details of the CK plan. Median collimator size for all treatment plans was 20 mm. Since CK plans are generated as non-isocentric by inverse solution iterative process, a physicist can assign individual fine tuning to add or remove doses of beams. Our current protocol is to prescribe a total dose of 36.25 Gy in 5 fractions (7.25 Gy per fraction) to PTV and maximum 32 Gy to the bladder and rectum as critical organs. Due to the steep dose gradient, we did not define doses to femoral heads. Maximum dose, minimum 70% isodose coverage to PTV, beamlet numbers and treatment time were taken into consideration during evaluation of the plans. The maximum dose of uretha was defined at 41–42 Gy. Fig. 4 presents planning system of CK for a sample patient.
Table 3.
The CyberKnife plan dose prescription index.
| Structure | Type | Objective |
|---|---|---|
| PTV | Target | Max Dose (cGy): 48 |
| Min Dose: 36.25 | ||
| Bladder | OAR | Max Dose (Gy): 32 |
| Rectum | OAR | Max Dose (Gy): 32 |
| Urethra | OAR | Max Dose (Gy): 41.69 |
Fig. 4.
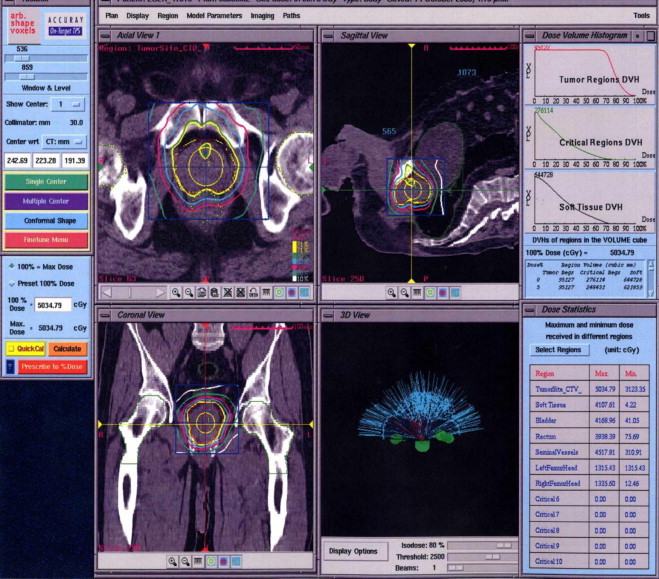
Treatment plan screen of the CyberKnife.
The total planning time for IMRT was approximately 5 h including image transfer, fusion of image sets, delineation of ROIs, placement of beams, dose prescriptions and computer calculation to find the best solution. The verification of IMRT plan should also be added to this time. Although there are different methods and procedures that can be used,11 patient set-up accuracy of prostate cases is performed using online correction by correlation with bony landmarks between DRRs and portal imaging taken every other day.24 Absolute dose verification of plans was done by ion chamber measurements using an IMRT phantom (PTW Freiburg, Germany) and relative dose distribution comparison on an array dosimeter.
CK plans were generated in approximately 3–4 h, including all processes to find an optimum plan without having to check the plan individually for each patient. Therefore, monthly and weekly quality assurances of CK consist of phantom treatment plans verification to evaluate robot and linear accelerator performance. During treatment, CK is capable to correct patient set-up in five dimensions, such as translations and rotations, by tracking at least 3 fiducials which were pre-installed into patient's prostate. The uncertainty of tracking fiducials combines the effects of all errors originating from image guidance, indicating the accuracy of the robot and the accuracy of target localization in treatment planning process.
2.3. Comparison of treatment plans
We prescribed the maximum PTV dose as 5% higher than 74 Gy and minimum PTV dose as 1% higher than 74 Gy to achieve 95% coverage of PTV with 74 Gy on IMRT plans, while keeping the doses to the bladder and the rectum at the level exceeding 70 Gy as ≤10%.
Fig. 6 presents a sample final IMRT plan of a patient. Sixteen IMRT and CK plans were performed for comparison in terms of conformity (CI) and homogeneity indices (HI),25,26 and percentage doses of 100%, 66%, 50%, 33% and 10% volumes of the bladder and rectum. These volumes were denoted as V100, V66, V50, V33 and V10. No comparison was done for femoral heads since doses at femoral heads were negligible.
Fig. 6.
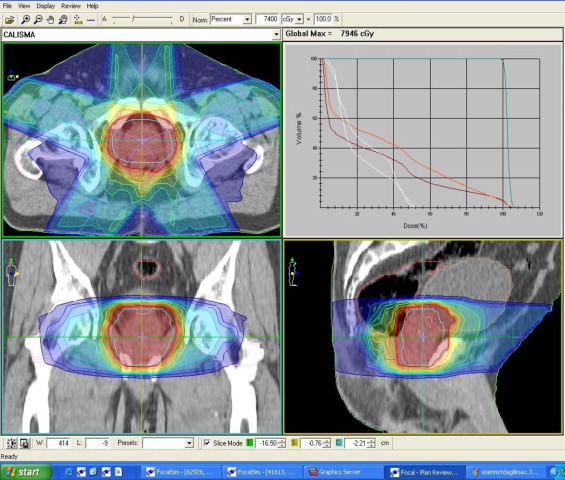
IMRT plan of the patient.
We defined the conformity and homogeneity index equations as follows:
| (1) |
| (2) |
where VRI is the volume of prescribed dose for PTV, TV is the total volume of PTV, Imax is the maximum dose and RI is the prescribed dose of PTV. Differences were reported using Student's t-test with statistical significance at the p < 0.05 levels (two tailed).
3. Results
CK and IMRT plans for 16 localized prostate patients were generated according to the clinical acceptable criteria to cover PTV sufficiently. For the purpose of this study, we evaluated plans with the consideration of physical and dosimetric components of treatment without clinical inspection. As different margins had been applied while creating CTV volume for both IMRT and CK plans, different CTV volumes were obtained for both plannings. Whereas median CTV volume for IMRT plan was 132.5 cm3, 88.5 cm3 was found for the CK plan. Fig. 5 presents PTV volumes of patients which were delineated IMRT and CK plans with different margins
Fig. 5.
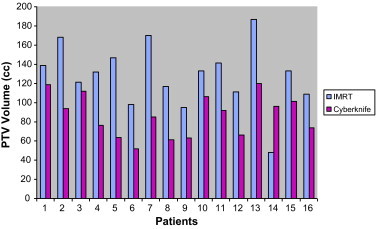
PTV volumes of patients.
Figs. 6 and 7 show dose distributions of CK and IMRT for the same patient. CK plans were normalized to median 75% isodose lines to obtain 95% coverage of PTV. Dose distributions of the two plans reveal that CK has a higher dose gradient than IMRT. The DVHs comparing IMRT and CK for the same patient are shown in Fig. 7.
Fig. 7.
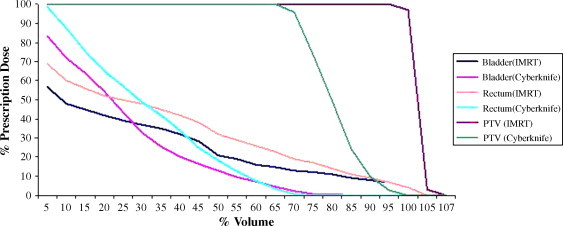
DVH comparison of the CyberKnife and IMRT plans for a patient.
Table 4 shows statistical results of the two plans. The median conformity indices of PTV for IMRT and CK plans were 0.94 and 1.23 (p < 0.001), respectively. Both systems have a very good ability to create highly conformal volumetric dose distribution. We also compared the homogeneity of the two plans and observed that the median homogeneity value of IMRT plans is closer to 1 than CK plans into the target. The median homogeneity indices of PTV for IMRT and CK plans are 1.08 and 1.33 (p < 0.001), respectively. The inherent property of CK plan is the heterogeneous dose distribution across the PTV. This property may enable us to modify the dose distribution within PTV and to intensify the dose especially in hypoxic regions.
Table 4.
Median percent doses for bladder and rectum volumes in all patients. V10, V33, V50, V66 and V100 denote percentage volumes of organs that 10%, 33%, 50%, 66% and 100% of volumes, respectively. Table presents CI, HI and maximum and minimum doses for PTV as a percent of prescription dose.
| Median doses |
p Value | ||
|---|---|---|---|
| IMRT | CK | ||
| %Bladder volume | |||
| V10 | 96.25% | 47.80% | <0.001 |
| V33 | 33.75% | 23.75% | 0.047 |
| V50 | 5.40% | 13.40% | 0.432 |
| V66 | 3.45% | 8.05% | 0.198 |
| V100 | 0.85% | 0.10% | 0.02 |
| %Rectum volume | |||
| V10 | 96.30% | 57.05% | <0.001 |
| V33 | 59.00% | 35.10% | <0.001 |
| V50 | 38.48% | 27.45% | 0.019 |
| V66 | 12.73% | 20.95% | 0.287 |
| V100 | 2.05% | 2.60% | 0.049 |
| PTV | |||
| CI | 0.94 | 1.23 | <0.001 |
| HI | 1.08 | 1.33 | <0.001 |
| Maximum percentage dose | 108% | 133% | |
| Minimum percentage dose | 95.30% | 86% | |
For normal tissues, we evaluated the percentage doses of 10%, 33%, 50%, 66% and 100% volumes of the bladder and rectum in IMRT and CK plans. Significant differences in the rectal doses delivered to V10, V33, and V50 were observed in favor of CK plans with p values of <0.001, <0.001 and 0.019, respectively. The percentage doses for V66 and V100 of the rectum were larger for CK plans. They were 12.73% and 2.05% for IMRT and 20.95% and 2.60% for CK, respectively. Bladder doses for V10 and V33 were significantly lower for CK plans. They were 96.25% and 33.75% for IMRT and 47.80% and 23.75% for CK, respectively (p < 0.001 and p = 0.047, respectively). Lower percentage doses were observed for V50 and V66 of the bladder in IMRT. They were 5.4% and 3.45% for IMRT and 13.4% and 8.05% for CK, respectively. Results of the analyses of all patients’ data are shown in Fig. 8.
Fig. 8.
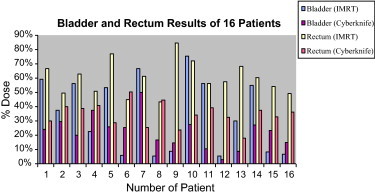
Bladder and rectum results for all patients.
4. Discussion
In our current study, we demonstrated the physical and dosimetric comparison of IMRT and CK plans for localized prostate cancer. We evaluated the plans considering target coverage, conformity and homogeneity indices, and the bladder and rectum doses.
Our results indicated that the conformity and homogeneity of IMRT plans were better than CK plans. Although one of the characteristics of IMRT is the ability to create a dose volume with a very high conformity index, radiation doses to small volumes of the bladder and rectum were higher than CK. CK was superior to IMRT in protecting the rectum and bladder, especially in high dose areas as shown in Fig. 8. Depending on the nature of CK, beams reach the patient from 1200 points around the patient. Larger volumes of the bladder and rectum received low radiation dose in the CK plan. This should be considered when total doses of the rectum and bladder are calculated.
We observed that CK plans had inhomogeneous dose distributions compared to IMRT plans. The classical approach of radiotherapy is to cover the PTV with prescribed dose while keeping homogeneity within the PTV. However, some areas in the prostate may require higher doses than other tumor containing areas because of the hypoxic tumor sites. CK plans have the advantage to give radiation dose into these areas when hypoxic areas are defined.
Prostate motion and margins have been investigated extensively. It was shown that the marked movement of the prostate is in the anterior-posterior direction.14,27,28 We used a 1 cm margin for all directions, except posterior which was 0.5 cm for PTV, and aimed to create an IMRT plan covering 95% of PTV by prescribed dose while keeping account of prostate motion and set-up errors. All IMRT patients were treated with full bladder to minimize prostate motion and all patients treatment position accuracies were evaluated with daily portal imaging by bony structures superposed with DRR and portal imaging. We did not use fiducials for IMRT patients. Therefore, increased margins in IMRT plans could cause an increase of the volume of the bladder and rectum. PTV was created with 0.5 cm margin in all directions for CK plan and we implemented 4 fiducials into the prostate to follow prostate motion during treatment by taking on-line orthogonal X-ray images. Thus, CK has the advantage for accurate target localization by real time tracking and it can treat the patient with lower dose to the bladder and rectum with reduced PTV margin. We show that CK system has some advantage to treat early stage prostate cancer and Bindhu et al.29 conclude that a CK radiosurgery system offers an invaluable solution to the treatment of selective tumours/lesions located close to critical structures, salvage of recurrent and metastatic lesions and potential of treatment of selective early stage malignancies like the carcinoma of the prostate and lung.
Despite the improved conformity with IMRT, the application is limited by the treatment set-up, target movement errors and long treatment days. The accurate delivery of radiotherapy for prostate cancer needs involvement of IGRT, 4DCRT or CK. Although we did not focus on the radiobiology of prostate cancer in our current study, there are several30 papers reporting that since the value of the α/β parameter for prostate tumors is low, it may mean a high sensitivity to hypo-fractionation. CK treatment protocol with hypo-fractionation might yield high tumor control probability.
5. Conclusion
In the present study, we show that the difference in PTV coverage between IMRT and CK plans is not very significant. Although the homogeneity index for IMRT plans is better than CK plans, CK system could give opportunity to evaluate the homogeneity considering the high dose within the prostate gland. We concluded that CK has many attributes that allow prostate treatment to be delivered very accurately and it can be an alternative to treat prostate efficiently. In our hospital, we currently perform IMRT and CK treatments for early stage localized prostate cancer and we plan to publish results of these treatments to compare them with other treatment options in terms of PSA level and side effects.
References
- 1.Ashman Jonathan B., Zelefsky Micheal J., Hunt Margie S., Leibel Steven A., Fuks Zvi. Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(3):765–771. doi: 10.1016/j.ijrobp.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 2.Luxton Gary, Hancock Steven L., Boyer Arthur L. Dosimetry and radiobiological model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2004;59(1):267–284. doi: 10.1016/j.ijrobp.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Vlachaki Maria T., Teslow Terrance N., Amosson Chad, Uy Nathan W., Ahmad Salahuddin. IMRT versus conventional 3DCRT on prostate and normal tissue dosimetry using an endorectal balloon for prostate immobilization. Med Dosim. 2005;30(2):69–75. doi: 10.1016/j.meddos.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.James H.V., Scrase C.D., Poynter A.J. Practical experience with intensity-modulated radiotherapy. Br J Radiol. 2004;77:3–14. doi: 10.1259/bjr/14996943. [DOI] [PubMed] [Google Scholar]
- 5.Kara Bucci M., Bevan Alison, Roach Mack., III Advances in radiation therapy: conventional to 3D, to IMRT, to 4D, and beyond. CA Cancer J Clin. 2005;55:117–134. doi: 10.3322/canjclin.55.2.117. [DOI] [PubMed] [Google Scholar]
- 6.van de Bunt Linda, Van der Heide Uulke A., Ketelaars Martijn, de Kort Gerard A.P., Jürgenliemk-Schulz Ina M. Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: the impact of tumor regression. Int J Radiat Oncol Biol Phys. 2006;64(1):189–196. doi: 10.1016/j.ijrobp.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 7.De Meerleer Gert, Vakaet Luc, Meersschout Sabine, Villeirs Gert, Verbaeys Antony, Oosterlinck Wim. Intensity modulated radiotherapy as primary treatment for prostate cancer: acute toxicity in 114 patients. Int J Radiat Oncol Biol Phys. 2004;60(3):777–787. doi: 10.1016/j.ijrobp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 8.De Meerleer Gert, Vakaet Luc, De Gersem Werner R.T., De Wagter Carlos, De Naeyer Bart, De Neve Wilfried. Radiotherapy of prostate cancer with or without intensity modulated beams: a planning comparison. Int J Radiat Oncol Biol Phys. 2000;47(3):639–648. doi: 10.1016/s0360-3016(00)00419-3. [DOI] [PubMed] [Google Scholar]
- 9.Sethi Anil, Mohideen Najeeb, Leybovich Leonid, Mulhall John. Role of IMRT in reducing doses in dose escalation for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;55(4):970–978. doi: 10.1016/s0360-3016(02)04164-0. [DOI] [PubMed] [Google Scholar]
- 10.Zelefsky Micheal J., Fuks Zvi, Happertsett Laura, Lee Henry J., Ling C. Clifton, Burman Chandra M. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiat Oncol. 2000;5:241–249. doi: 10.1016/s0167-8140(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 11.Ezzell Gary A., Galvin James M., Low Daniel, Palta Jatinder R., Rosen Isaac, Sharpe Michael B. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT subcommittee of the AAPM radiation therapy committee. Med Phys. 2003;30(8):2089–2115. doi: 10.1118/1.1591194. [DOI] [PubMed] [Google Scholar]
- 12.Boehmer Dirk, Maingon Philippe, Poortmans Philip, Baron Marie-Hélène, Miralbell Raymond, Remouchamps Vincent. Guidelines for primary radiotherapy of patients with prostate cancer. Radiat Oncol. 2006;79:259–269. doi: 10.1016/j.radonc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Jones Andrew O., Kleiman Marc T. Patient setup and verification for intensity-modulated radiation therapy (IMRT) Med Dosim. 2003;28(3):175–183. doi: 10.1016/S0958-3947(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 14.Nederveen A.J., van der Heide U.A., Dehnad H., van Moorselaar R.J., Hofman P., Lagendijk J.J. Measurements and clinical consequences of prostate motion during a radiotherapy fraction. Int J Radiat Oncol Biol Phys. 2002;53:206–214. doi: 10.1016/s0360-3016(01)02823-1. [DOI] [PubMed] [Google Scholar]
- 15.Aubin M, Liu Y, Langen KM, Shinohara Katsuto, Anezinos Chris, Osofsky Melanie, et al. Set-up verification using portal images of implanted markers: an inter-observer study. In Abstract 44th annual ASTRO meeting, 2002.
- 16.Brenner David J. Hypofractionation for prostate cancer radiotherapy—What are the issues? Int J Radiat Oncol Biol Phys. 2003;57(4):912–914. doi: 10.1016/s0360-3016(03)01456-1. [DOI] [PubMed] [Google Scholar]
- 17.Fowler F., Ritter M.A., Chappell R.J., Brenner David J. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–2003. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 18.Galvin James M., Ezzell Garyy, Eisbrauch Avraham, Yu Cedric, Butler Brian, Xiao Ying. Implementing IMRT in clinical practice: a joint document of the American society for therapeutic radiology and oncology and the American association of physicists in medicine. Int J Radiat Oncol Biol Phys. 2004;58(5):1616–1634. doi: 10.1016/j.ijrobp.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Carlo Cavedon, Joseph Stancanello, Paolo Francescon, Stefania Cora, Paolo Scalchi,Emanuela Berna, et al. Spelized imaging techniques for cyberknife treatment planning. In Robotic radiosurgery, vol. 1; 2005. p. 81–94 [chapter 9].
- 20.Pollack Alan, Zagars Gunar, Starkschall Gergo, Antolak John A., Lee J. Jack, Huang Eugene. Prostate cancer radiation dose response: results of the M.D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 21.Zelefsky Micheal J., Fuks Zvi, Hunt Margie, Yamada Yoshiya, Marion Christine, Ling C. Clifton. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53(5):1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 22.Adams Elizabeth J., Convery David J., Cosgrove Vivian P., McNair Helen A., Staffurth John N., Vaarkamp Jaap. Clinical implementation of dynamic and step-and-shoot IMRT to treat prostate cancer with high risk of pelvic lymph node involvement. Radiat Oncol. 2004;70:1–10. doi: 10.1016/j.radonc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Livi Lorenzo, Paiar Fabiola, Banci-Buonamici Fabrizio, Gabriele Simontacchi, Beatrice Detti, Mauro Gacci. Localized prostate cancer treated with intensity-modulated radiotherapy. Tumori. 2006;92:201–206. doi: 10.1177/030089160609200302. [DOI] [PubMed] [Google Scholar]
- 24.Melian Edward, Mageras Gig S., Fuks Zvi, Leibel Steven A., Niehaus Anita, Lorant Helen. Variation in prostate position quantitation and implications for three-dimensional conformal treatment planning. Int J Radiat Oncol Biol Phys. 1997;38(1):73–81. doi: 10.1016/s0360-3016(97)00221-6. [DOI] [PubMed] [Google Scholar]
- 25.Wu Vincent W.C., Kwong Dora L.W., Sham Jonathan S.T. Target dose conformity in 3-dimensional conformal radiotherapy and intensity modulated radiotherapy. Radiat Oncol. 2004;71:201–206. doi: 10.1016/j.radonc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Feuvret Loic, Noel Georges, Mazeron Jean-Jacques, Bey Pierre. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64(2):333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Teh Bin S., Bastach Micheal D., Wheeler Thomas M., Maiet Wei-Yuan, Frolov Anna, Uhl Barry M. IMRT for prostate cancer: defining target volume based on correlated pathologic volume of disease. Int J Radiat Oncol Biol Phys. 2003;56(1):184–191. doi: 10.1016/s0360-3016(03)00085-3. [DOI] [PubMed] [Google Scholar]
- 28.Brenner David J., Hall Eric J. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43(5):1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 29.Bindhu J., Supe Sanjay S., Ramachandra Aruna. Cyberknife: a double edged sword? Rep Pract Oncol Radiother. 2010;15(4):93–97. doi: 10.1016/j.rpor.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soete G., Arcangeli S., De Meerleer G., Landoni V., Fonteyne V., Arcangeli G. Phase II study of a four-week hypofractioned external beam radiotherapy regimen for prostate cancer: report on acute toxicity. Radiother Oncol. 2006;80:78–81. doi: 10.1016/j.radonc.2006.06.005. [DOI] [PubMed] [Google Scholar]


